Making one-sided leaf measurements
Printable PDF: Making one-sided leaf measurements
(6800_Note-One-sided-leaf-measurement-18444.pdf)
Download this content as a pdf that can be saved to your computer or printed.
Standard chambers for the LI-6800 interact with and measure gas exchange from both the adaxial and abaxial sides of the leaf material enclosed in the chamber. Computed fluxes of CO2 (A) and H2O (E) as well as stomatal conductance (gsw) are a summation of interactions from both leaf surfaces. For some research questions, it may be desirable to isolate and measure gas exchange on a single side of the leaf.
One application for measuring a single side of the leaf would be to determine the ratio of stomata on the different sides of the leaf. An input to the equation for stomatal conductance is the ratio of stomatal density on the leaf surfaces (k, see equation C-9 of LI-6800 manual). Knowledge of the stomatal ratio for the species being studied allows for more precise computations of gsw when accounting for boundary layer conductance. One technique for measurement of the stomatal ratio is to measure gsw separately on both leaf surfaces.
The plumbing of the LI-6800 allows you to make one-sided measurements. However, there are a number of considerations when making these measurements. In this application note, we will describe the hardware kit available from LI-COR to make these one-sided measurements. Currently, this kit is only available for the 6 cm2 fluorometer (6800-01A) aperture. We will discuss good practices when making one-sided measurements and show some example data sets.
What’s what
The complete hardware kit (part number 9968-313) is shown in Figure 1‑1. It includes a gasket mounted to a 6 cm2 aperture sealed with Propafilm (part number 6568-646) and a needle valve assembly (part number 9968-312).

The gasket has over-molded tubing that allows air to interact with the excluded portion of the leaf, as well as an exhaust port to flow air to a secondary analyzer, if desired. The kit includes a proportioning valve that attaches to the air supply at the back of the head. Air is diverted to flush the air space adjacent to the side of the leaf not being measured.
The Propafilm isolates one side of the leaf, preventing gas exchange from one side from interacting with the LI-6800 gas exchange system by flushing the volume surrounding the excluded side of the leaf with reference air See Measurement considerations for details.
The leaf material being measured is used to block flow from interacting with the excluded half of the chamber. If the leaf does not fill the aperture, air will mix between the chamber halves and both sides of the leaf will be measured. A requisite of this kit is that the leaf material cover the entire aperture.
Installing the kit
The valve fits between the air supply tube and the LI-6800 head. The one-sided aperture goes into the upper or lower portion of the chamber. To install the kit, disconnect the air supply tube from the head: pull the quick-connect coupler. Install the proportioning valve and connect the air supply tube. Attach the 1/8" tube over the hosebarb on the valve. The valve can rotate on the body of the quick-connects. Turn it to access the hosebarb.
The white advanced polymer gasket is shipped with a square piece of protective transparent film. Remove it before taking measurements.
If you are installing the aperture in the lower chamber remove the leaf temperature thermocouple and replace it with a plug from the fluorometer spares kit. Or adjust it so it is out of the way of the film.
Measurement considerations
Below we describe some important considerations for measuring one side of a leaf.
Flushing the excluded side of the leaf
Gas exchange theory dictates that a difference in [CO2] between the atmosphere in the chamber on the measured side of the leaf (Cs) and the atmosphere surrounding the excluded portion of the leaf (Ca) will result in a change to the measured apparent assimilation rate. An experiment to demonstrate this was performed by measuring steady-state assimilation on a tobacco leaf over a range of [Ca] (Figure 1‑3). In this experiment, [Ca] was altered and data logged after a new steady-state is reached, ~1.5 minutes per data point.
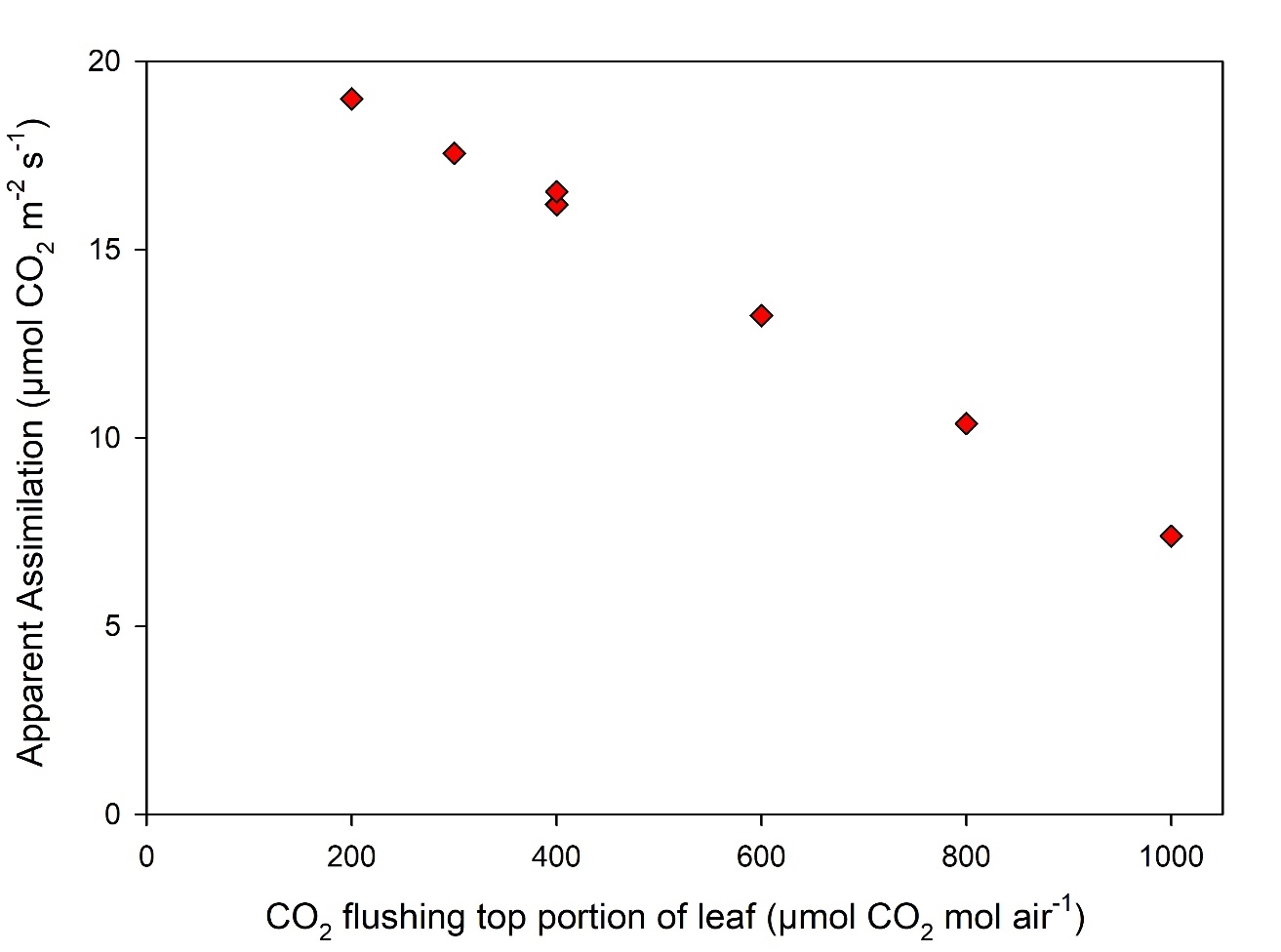
The example in Figure 1‑3 highlights the importance of maintaining Ca at a constant value even on the side of the leaf excluded in the measurement. The one-sided hardware kit includes provisions to flush the excluded portion of the leaf with reference air. The volume of the air-space surrounding the excluded portion of the air is the area of the gasket (6 cm2) × the height of the gasket (~0.5 cm) or 3 cm3. This small volume can be rapidly flushed with a low flow rate. For example 100 µmol s-1 (~2.4 cc s‑1) results in flushing the volume in <2 seconds, sufficiently fast for measurements in the LI-6800 system.
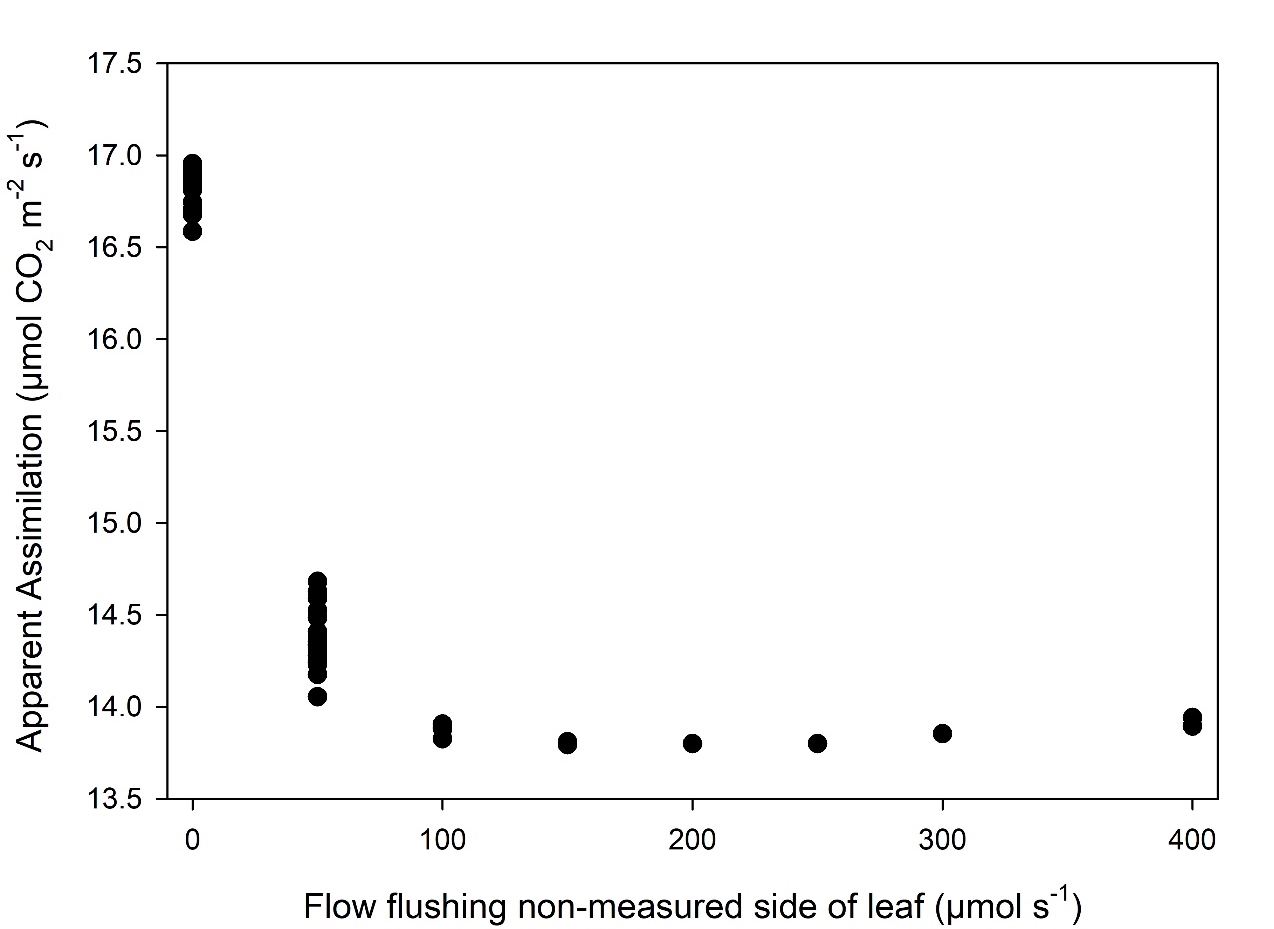
The proportional valve in the one-sided kit allows you to adjust the amount of air being diverted to flush the excluded portion of the leaf. Care should be taken to leave enough air flow through the reference path (see Measurement Protocol). As long as the cuvette is flushed to ensure Ca ≈ Cs, the results for computed assimilation are independent of flow rate to flush the excluded side of the leaf (Figure 1‑4). Our testing finds 100 – 150 µmol s-1 is sufficient to properly flush the volume. Flows should be adjusted to ensure ample flow to the sample and reference analyzers without compromising the measurement.
Transmittance
The one-sided kit aperture is covered by a layer of propafilm which acts as a neutral-density filter with respect to light intensity. When the top portion of the cuvette is being excluded (recommended, see Leaf Temperature), the propafilm will be between the light source and the leaf. Consequently, there will be a drop in light intensity in the plane of the leaf for a given light output by the LEDs. This can be accounted for by changing the leaf transmittance value. For the propafilm included with the kit (part number 250-01885), the change in transmittance has been measured at 0.82 for the red, blue and far-red LEDs in the 6 cm2 fluorometer aperture. This can easily be changed in the LI-6800 software when setting up measurements (see Measurement Protocol). If the propafilm is damaged and/or replaced with a different material, be sure to measure the transmittance of the new material. These measurements can be performed by removing the cuvette bottom and replacing with the external LI-190R quantum sensor, such that it is approximately in the plane of the leaf. A layer of poster tack around the sensor helps to keep it in place and fill in gaps. Light intensity measured by that sensor should then be measured with the standard aperture (no propafilm) and again with the one-sided aperture with propafilm. The ratio between the two measurements is the transmittance factor to use with that gasket in place on the top aperture window.
Leaf Temperature
The leaf temperature in the LI-6800 is measured by a thermocouple in contact with the bottom half of the leaf. If excluding the top portion of the leaf, this measurement is not impacted and no changes are required. However, if excluding the bottom portion of the leaf, the propafilm prevents the thermocouple from contacting the leaf and leaf temperature can no longer be measured. In that scenario, use energy balance to compute leaf temperature.
Measurement Protocol
This section describes how to set up for measurements using the one-sided kit.
- Decide if the top or the bottom of the cuvette is being excluded and install the one-sided aperture into the upper or lower cuvette.
- This decision has several important consequences on measurement quality and on setting up the software. A primary consideration should be the measurement of leaf temperature, which is a direct input to stomatal conductance (gsw) and inter-cellular CO2 concentration (Ci). If these parameters are of interest, excluding the top portion of the cuvette may be a better choice as this allows for the measurement of leaf temperature with the thermocouple.
- Set up the air-flow.
- Begin with the valve of the one-sided kit completely closed. Set up the desired cuvette flow rate. Monitor Flow_r in the Environment > Flow screen. For this application, Flow_r should be ≥550 µmol s-1 with the valve closed. If it is not, pump speed can be increased. Once Flow_r is stable, slowly open the valve on the one-sided kit until Flow_r decreases by ~150 µmol s-1. A precise flow rate to flush the excluded portion of the leaf is not necessary as it is not used in any calculations. Enough flow is needed to ensure that volume is rapidly flushed and [CO2] and [H2O] in the air surrounding the leaf remain constant and near chamber conditions. The flow should be periodically checked to make sure the excluded half is being flushed.
- If the top half is being excluded, the value for Transmittance must be set in the Environment > Light screen. First, clear the box labeled Source is attached to 6800-01A Fluorometer w 6 cm2 aperture, then change Transmittance to 0.82 (or as measured if the propafilm has been changed).
- Set stomatal ratio to 0 for one-sided measurement.
- In the Constants > Gas Exchange tab, set K = 0.
- If the bottom half of the chamber is excluded, leaf temperature thermocouple cannot touch the leaf surface and energy balance must be used to calculate leaf temperature.
- Go to Constant > Leaf Temperature and choose the Energy Balance option.
- (Optional) If alternating between top and bottom position then you may want to consider enabling Prompt on manual log on the Log Options. This will prompt you to change the constants for stomatal ratio and leaf temperature.
- Set up all environmental conditions as desired and begin making measurements as normal. Be sure to occasionally verify the flow rate to the excluded portion of the cuvette.
Example Data
Example experiments demonstrate the utility of the kit in survey measurements and response curves.
Survey Measurement
Survey measurements were made on leaves of Phaseolus coccineus grown under polytunnel conditions in the UK. For this experiment 3 sets of survey measurements were taken from 10 leaves, each set containing one whole chamber measurement, one measurement of the abaxial side only and one measurement of the adaxial side. Chamber conditions were: PAR 600 µmol m-2 s-1, 90% red, flow 600 µmol s-1, CO2sample 400 µmol mol-1, leaf VPD 1.5 kPa, fan speed 10,000 rpm, exchanger temperature 22 °C. For the one-sided measurements, the flow flushing the excluded side was 150 µmol s-1. Results are shown in Table 1‑1.
Light Response Curves
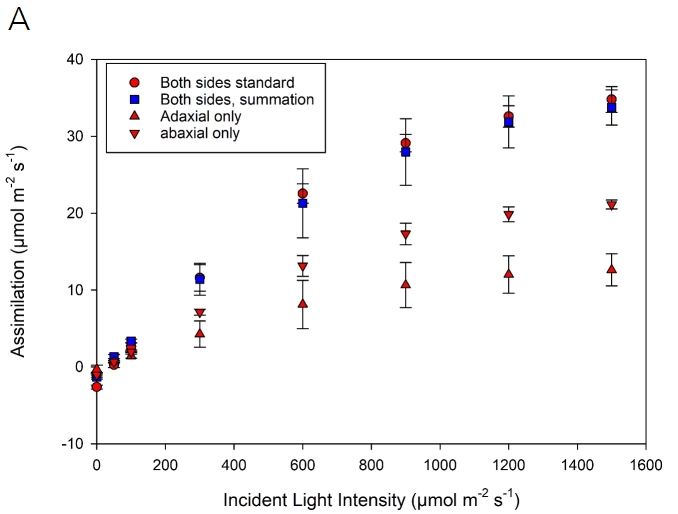
Light response curves were also performed on leaves of Zea mays grown in the LI-COR greenhouse. For this experiment, two LI-6800 instruments were utilized, one each with the standard fluorometer and the one-sided measurement kit. Three individual leaves were measured for light response curves. The two sensor heads were clamped onto the same leaf directly adjacent to minimize leaf variation. On each leaf, two light response curves were run, measuring the adaxial and abaxial portions individually. Data presented (Figure 1‑5) are mean values from three curves per leaf side and six curves from the standard cuvette. Before each curve, the leaf was acclimated for ~1 hour at 1500 µmol m-2 s-1 light intensity. Chamber conditions were flow of 500 µmol s-1, CO2sample 400 µmol mol-1, leaf VPD 1.5 kPa, fan speed 10,000 rpm, leaf temperature 27 °C (using energy balance when the bottom portion of the leaf is excluded). For the one-sided measurements, the flow flushing the excluded side was 150 µmol s-1. An auto-program was run to reduce incident light intensity with a data point logged three minutes after a change in light. The following values of light intensity were measured: 1500, 1200, 900, 600, 300, 100, 50, 0 µmol m-2 s-1.
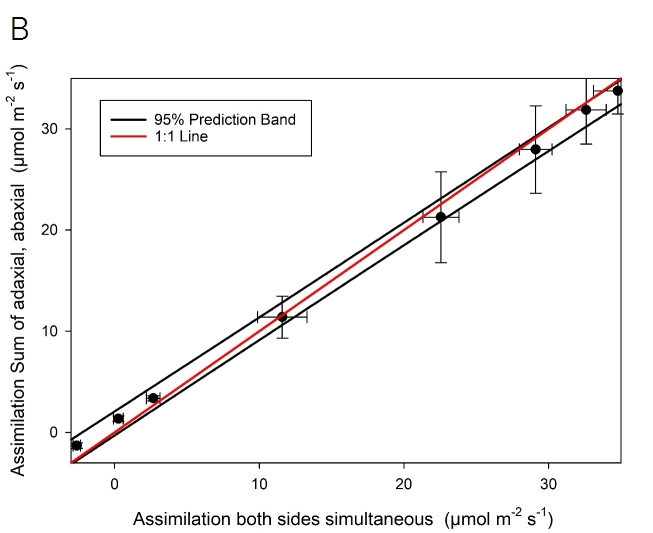
Assimilation rates measured on the adaxial and abaxial leaves separately largely agree with the measurement in the standard cuvette (Figure 1‑5). With adequate flushing of the excluded portion of the chamber, the measurement of a single side of the leaf can be performed. These data suggest ~60% of stomates are found on the abaxial side of the leaf, with ~40% on the adaxial side, in agreement with typical stomatal ratios approaching 0.5 for grass species.