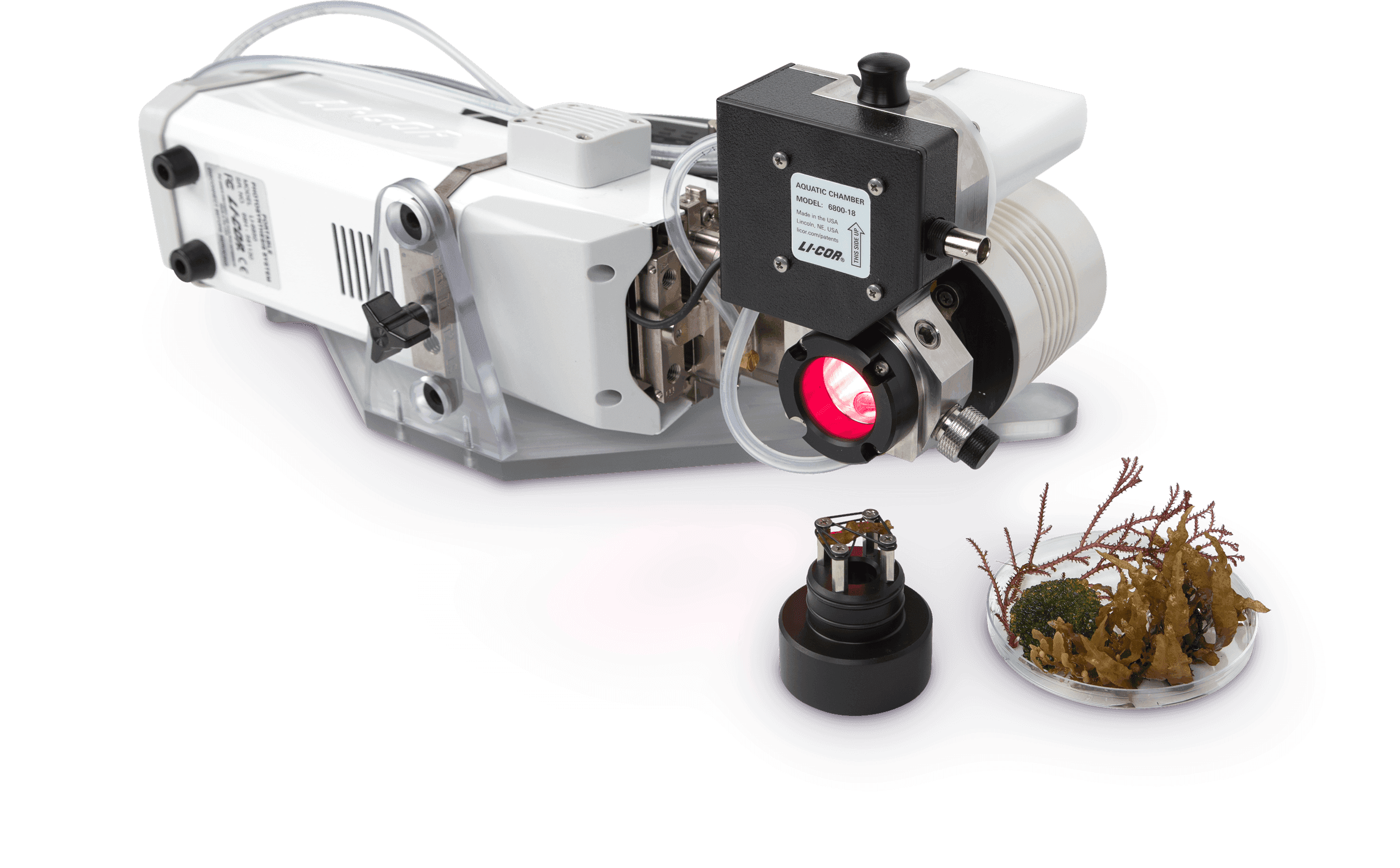
The 6800-18 Aquatic Chamber is used to measure steady-state carbon assimilation and chlorophyll a fluorescence from algal suspensions, coral, macro algae, and sea grasses using the LI-6800 Portable Photosynthesis System, the preferred photosynthesis system for terrestrial plant research.
Get a QuoteA recent publication in Algal Research describes direct measurements of carbon uptake and chlorophyll a fluorescence using the 6800-18 Aquatic Chamber.
For the study, authors Jason Hupp, Johnathon McCoy, both of LI-COR; Dr. Allan Milligan of Oregon State University; and Dr. Graham Peers of Colorado State University used the instrument to measure CO2 exchange from an aqueous solution of suspended cells at the same time that they examined chlorophyll fluorescence.
Read the PaperLearn how the new 6800-18 Aquatic Chamber measures CO2 exchange and chlorophyll a fluorescence from aquatic samples.
Watch Dr. Phillip Davey’s comments on the Aquatic Chamber, starting at 34:50.
Three novel applications described by Dr. Davey include:
“This chamber has many possibilities beyond algae, we are excited to explore coral, bryophytes, lichens and other organisms that are in solution or require constant wetting.”
— Dr. Tracy Lawson
Aquatic Chamber Beta Tester
Professor – Plant Physiology
University of Essex, Colchester, UK

The LI-6800 is trusted by leading researchers and institutions around the world to measure carbon assimilation (A) and pulse-amplitude modulated (PAM) chlorophyll a fluorescence in terrestrial plants. With high precision CO2 and H2O gas analyzers and automated system controls, the LI-6800 is used to test novel hypotheses at the forefront of photophysiology research.
The NEW 6800-18 Aquatic Chamber extends these capabilities to samples that must remain immersed in water or surrounded by humid air, enabling researchers to explore questions related to photosynthesis of algae in aquatic suspension, coral, and macrophytes.
In contrast with typical oxygen-based measurements of algal photosynthesis, where photosynthesis is derived from the change in O2 concentration over time, the LI-6800 is an open, flow-through, steady-state gas exchange system where CO2 and O2 concentrations are constant during the measurement.
In the 6800-18 Aquatic Chamber, the carbon assimilation rate is determined from the mass balance of an air stream before and after it interacts with a liquid sample. The CO2 and water vapor concentrations of the air stream are measured by a pair of high-precision infrared gas analyzers (IRGA). Assimilation is calculated from the concentration differences and flow rate:
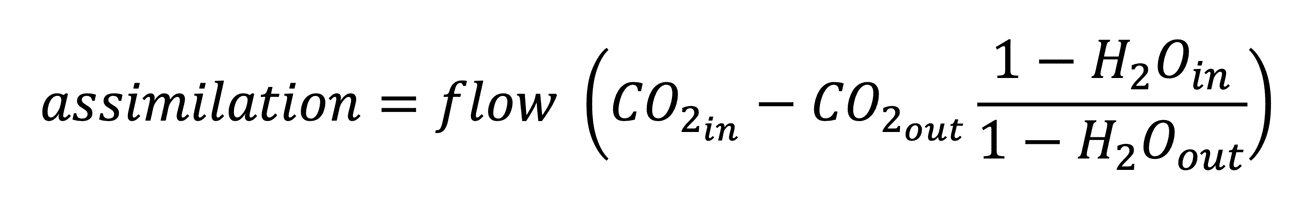

Fundamentally, this mass balance gives the flux of CO2 between the liquid sample and the cuvette headspace. The flux is coupled to the true biological carbon assimilation rate by mass transfer at the air-liquid interface and the kinetics of the carbonate system for the aquatic sample.
When normalized to cell density, mass, or chlorophyll content, the aquatic chamber provides measurements as µmol CO2 cell-1 s-1, µmol CO2 mg-1 s-1, and µmol CO2 µg-1 s-1, respectively.
A carefully controlled aeration scheme ensures that the mass transfer coefficient is not limiting and that the measured flux represents the biological assimilation rate of the sample. To keep the carbonate system at a steady state during measurements, carbonic anhydrase (CA) is added to the sample media for rapid hydration of CO2 and interconversion with bicarbonate (HCO3–) .
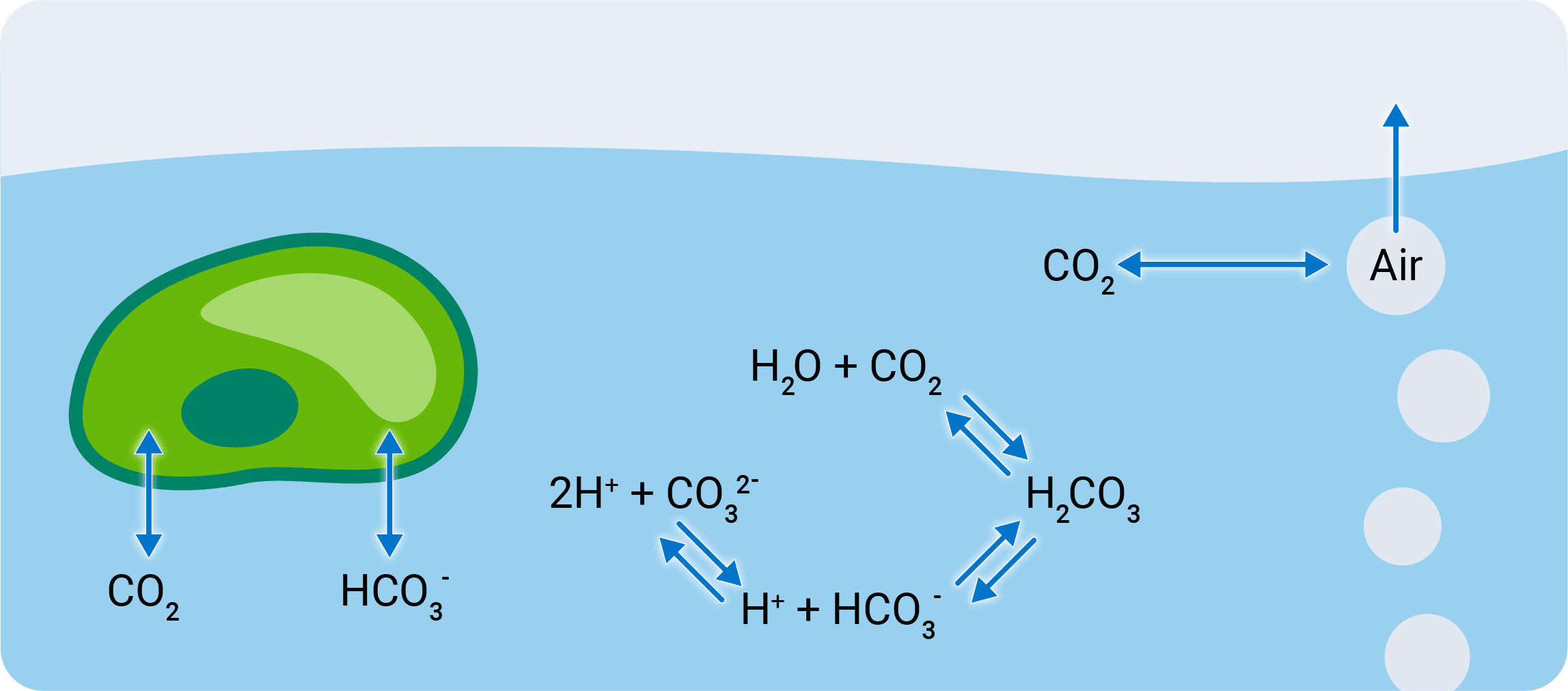
The LI-6800 precisely controls environmental conditions of the sample, including the CO2 concentration in headspace air and the light environment according to your preferences.
These features enable time-series data collection on a sample while environmental conditions are kept stable. This ensures that the measured biological response is in the context of known, steady-state conditions.
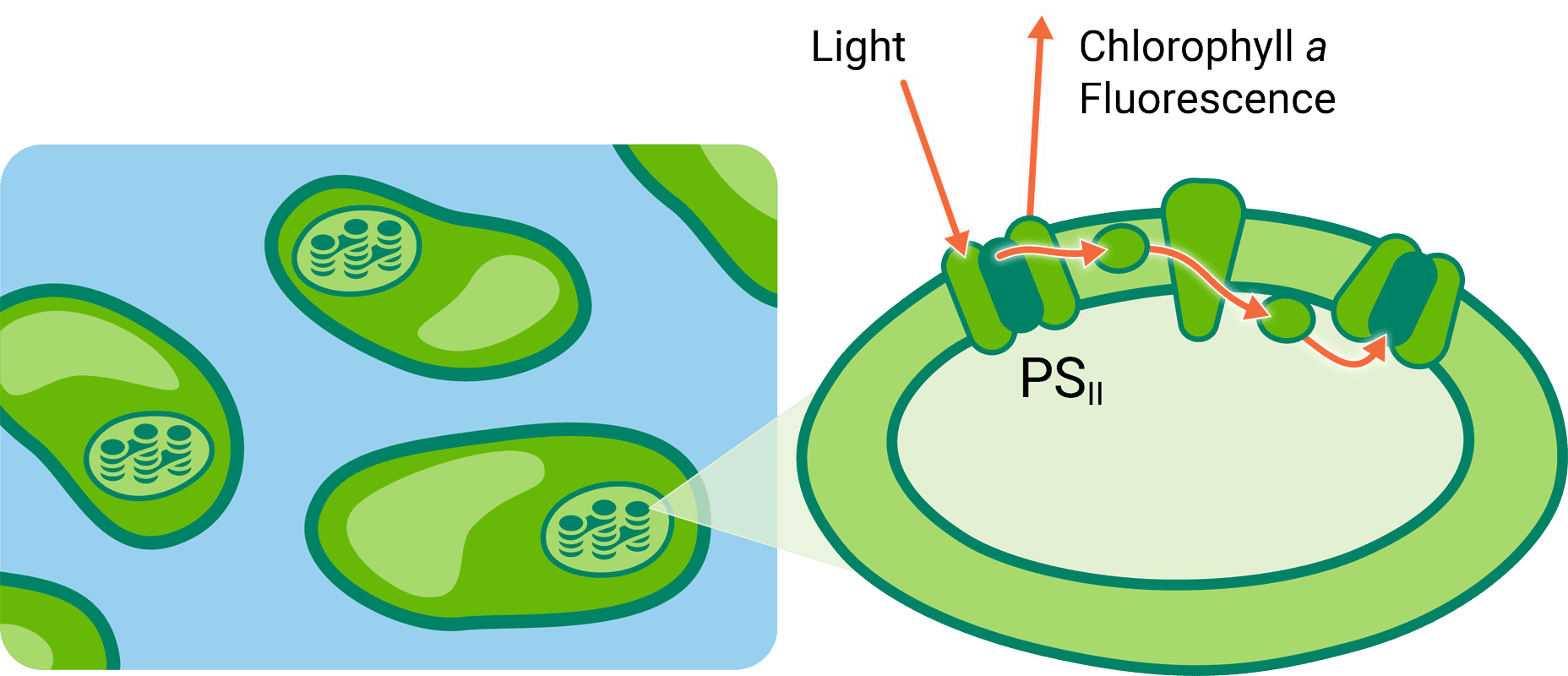
Simultaneous measurements of CO2 gas exchange and PAM chlorophyll a fluorescence of an aquatic sample provide a more complete impression of photosynthetic processes than either technique alone. CO2 exchange measurements are indicators of the photosynthetic interaction between algae and dissolved inorganic carbon in solution. Chlorophyll a fluorescence is an indicator of the light reactions of the organisms.
When combined, they reveal more about the photochemistry of algae than either technique alone.
From basic irradiance response measurements to complex multifactor experiments, the aquatic chamber can be used to evaluate biological responses to different environmental conditions.
Figure 3 shows assimilation measurements on Chlorella at ambient oxygen (21%) and low oxygen (0.5%) in response to light (Q). The CO2 concentration entering the chamber was held constant at 400 µmol mol-1 by the LI-6800 system. Air with 21% oxygen was ambient; low-oxygen air was from a tank of 0.5% oxygen balanced in air. Chamber temperature was held constant at 25 °C using an external water bath. Cells were measured in a saltwater media at 17 ppt salinity.

When O2 concentrations are reduced in solution, the likelihood of oxygenation of RuBP by RuBisCO—the first step of photorespiration—is reduced, and the overall efficiency of carbon assimilation relative to energy capture increases. There is little impact on the efficiency of PSII, shown here by the near identical behavior of ΦPSII. Photorespiration impacts the strength of electron sinks such that 1-qL may be expected to decrease some with an increase in photorespiration (Figure 4).

Active regulation of a portion of NPQ related to cyclic electron flow balances the energetic products of photochemistry, ATP, and NADPH—so as photorespiration is suppressed at low oxygen, down regulation of NPQ is observed (Figure 5).

The photosynthetic response to CO2 was measured on Monoraphidium at ambient O2 concentration and 700 µmol photons m-2 s-1 light intensity. The CO2 concentration entering the chamber was controlled at different setpoints during these measurements. Chamber temperature was held constant at 25 °C using an external water bath. Cells were measured in a freshwater media buffered to pH 7.0 with a TRIS buffer.
The photosynthetic response to CO2 is non-linear in nature (Figure 6). At low concentrations, CO2 is limiting, and assimilation changes proportionally to the concentration.


With the sample adapter, the aquatic chamber can be used to measure the photosynthetic response of samples that must remain in humid air, as well as samples that are too large to fit into the upper chamber opening, such as coral. Figure 8 shows the irradiance response of Sargassum as measured with the aquatic chamber.




A novel approach to measuring carbon assimilation and chlorophyll a fluorescence in algal suspensions
Read the Whitepaper
Using the 9968-338 Sample Adapter Kit with the 6800-18 Aquatic Chamber
Read the Full ReportThe LI-6800 is a well-developed system for photosynthesis research. It supports advanced configurations, automated controls, simple file management, and other features that let you focus on research.
Specifications subject to change without notice.
View specifications for the full LI-6800 Portable Photosynthesis System
The aquatic chamber can be purchased as a complete stand-alone system, as a new chamber and light source for an existing LI-6800 system (LI-6800 System required), or as a chamber only (LI-6800 System and fluorometer required).
Designed for the primarily aquatic researcher, this package includes the LI-6800 System with console, sensor head, cable assembly, 6800-01A Fluorometer, and 6800-18 Aquatic Chamber. Accessories include the Spares Kit, Power Supply, two batteries, Silica gel, Soda lime, and CO2 cartridges.
Get a Quote for the LI-6800AQIncludes the 6800-01A Fluorometer and the 6800-18 Aquatic Chamber. This package is for customers who already have an LI-6800 System and still need a 6800-01A Fluorometer or want a specific 6800-01A Fluorometer for their aquatic work to avoid routine switching back and forth to the leaf level measurements.
Get a Quote for the 6800FAQLI-6800 Aquatic Chamber for measuring Photosynthesis and Respiration in an aquatic solution. Must be used with the LI-6800 Portable Photosynthesis System and 6800-01A Fluorometer. Includes the Spares Kit.
Get a Quote for the 6800-18Aquatic Chamber Adapter Assembly for 6800-18. For use with samples such as sea grasses, macro algae, coral and bryophytes.
Back to Chambers and Light Sources