System Description
This section serves to acquaint you with the LI-6400XT’s operating principle, major components, and how it computes gas exchange quantities.
An Open System
Measuring Differentials
The LI-6400 is an open system, which means that measurements of photosynthesis and transpiration are based on the differences in CO2 and H2O in an air stream that is flowing through the leaf cuvette (Figure 1‑1).
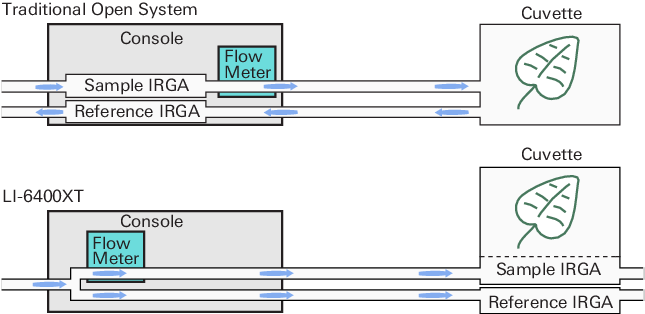
The LI-6400 improves upon traditional open systems by having the gas analyzers in the sensor head. This eliminates plumbing-related time delays, and allows tight control for responding to leaf changes. For example, if stomata close, the control system immediately detects the drop in water vapor and can compensate. Similarly, a sudden change in light level will cause an immediate change in photosynthetic rate, which will be detected as a change in the CO2 concentration. The speed of detection is not a function of the system’s flow rate, as in traditional systems, since the sample IRGA is in the cuvette.
There is a second advantage of having the IRGAs in the sensor head. The traditional system has the potential for concentration changes (because of water sorption and CO2 diffusion) as the air moves from the reference IRGA to the chamber, and again from the chamber back to the sample IRGA. This is not a problem for the LI-6400, because the IRGA measurements are made after the air has traveled through the tubing.
The Air Supply
One strength of an open system is that the incoming air stream can be conditioned. That is, its humidity, CO2 concentration, temperature, etc. can be established by some means prior to entering the system.
Regardless of what is done to the incoming air, however, one thing is crucial:
Incoming concentrations must be stable.
This is especially true for CO2, where the potential for fluctuations is huge (your breath, for example, is typically 30,000 μmol CO2 (mol air)-1. Fluctuations in incoming concentration will cause concentration differences to be very erratic.
Leaks
“Pressure inside the leaf chamber is slightly above ambient to ensure that leaks are outward, so outside air does not enter the chamber and affect the CO2 and H2O concentrations.” This argument for the advantage of an open system has been made for a long time, and it’s true - as far as it goes. There is another process at work: diffusion. CO2 will always diffuse from higher concentrations toward lower, even against a pressure gradient, and is only limited by the material through which it is moving. The black neoprene gaskets that we use on the LI-6400 (except for the light source) have the lowest diffusivity to CO2 of all the gasket material we have tested, but it’s not perfect. Diffusion effects are measurable at low flow rates when there is a large concentration difference between chamber and ambient. See Diffusion Leaks for more details.
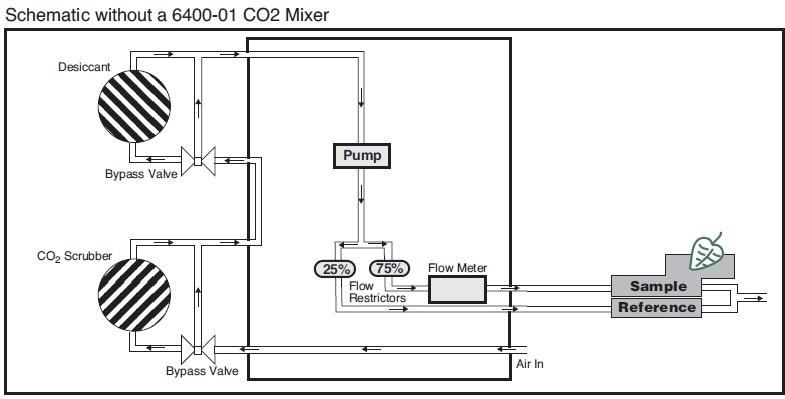
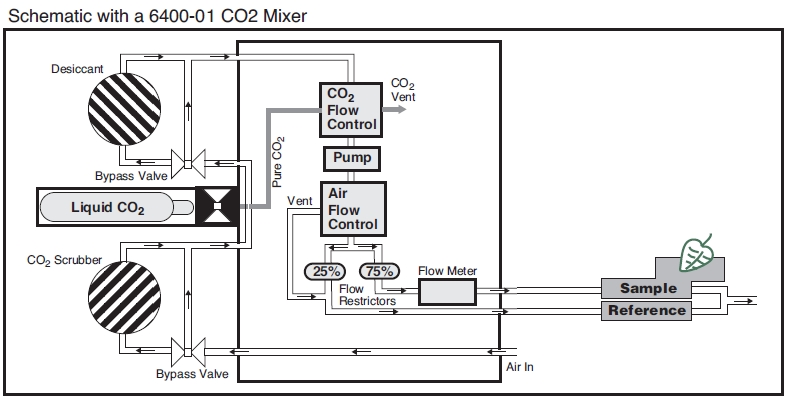
The Flow Schematic
The LI-6400 provides mechanisms for modifying the incoming air’s CO2 and H2O concentrations (Figure 1‑2). There are chemical tubes for scrubbing CO2 and H2O, and air can be diverted through these tubes in any proportion desired. CO2, however, is best controlled by scrubbing all of it from the incoming air, and using the 6400-01 CO2 mixer to inject just enough CO2 to provide a stable concentration at the desired value. If the 6400-01 is not part of your system, you will need to use a buffer volume. For a more complete discussion of buffer volumes, see Air Supply Considerations.
Humidity control in the leaf cuvette is achieved by regulating the flow rate that is going through the cuvette. In units without a CO2 mixer, the pump speed controls the flow, and in units with a CO2 mixer, a flow diverter regulates this flow.
Matching the IRGAs
The heart of the computation of transpiration and assimilation is the measurement of the concentration differences. Since these differences are measured by two independent infra-red gas analyzers, provisions must be made to check the IRGAs against one another. One way to do this is to compare the IRGAs when the leaf chamber is empty, and adjust one so that they match. Matching in this manner is a problem, however, because you don’t want to remove the leaf from the chamber in the middle of an experiment. The LI-6400 provides a mechanism to match the IRGAs without disturbing the leaf: it is called match mode, and it is illustrated in Figure 1‑3.
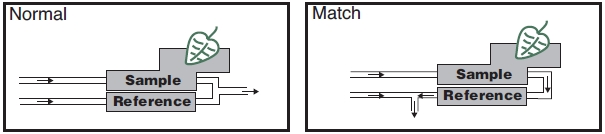
Match mode is something that you do at least once at the start of the day, and periodically throughout the day. Matching is important when the ΔCO2 or ΔH2O value is small (low rates, small leaf areas). For example, a 1 μmol mol-1 difference between the two CO2 IRGAs is trivial when the ΔCO2 is 75 μmol mol-1, but represents a significant error if the ΔCO2 is only 3 μmol mol-1.
Equation Summary
If you are not interested in the details of the LI-6400’s gas exchange calculations, you can safely skip this section.
The equations for net photosynthesis, transpiration, etc. are essentially those derived by von Caemmerer and Farquhar1. Note also that these equations represent the instrument’s defaults, and can be modified or replaced as desired by the user (Chapter 15 in the instruction manual).
Transpiration
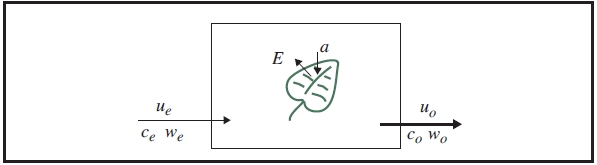
The mass balance of water vapor in an open system (Figure 1‑4) is given by
1‑1
where s is leaf area (m2), E is transpiration rate (mol m-2 s-1), ue and uo are incoming and outgoing flow rates (mol s-1) from the chamber, and we and wo are incoming and outgoing water mole fractions (mol H2O mol air-1). Since
we can write
1‑3
which rearranges to
The relationships between the terms in (1‑4) and what the LI-6400 measures are
where F is air flow rate (μmol s-1), Ws and Wr are sample and reference water mole fractions (mmol H2O (mol air)-1), and S is leaf area (cm2). The equation that the LI-6400 uses for transpiration is thus
Total Conductance to Water Vapor
The total (includes stomatal and boundary layer) conductance of the leaf gtw (mol H2O m-2 s-1) is given by
1‑7
where Wl is the molar concentration of water vapor within the leaf (mmol H2O (mol air)-1), which is computed from the leaf temperature Tl (C) and the total atmospheric pressure P (kPa)
1‑8
The function e(T) is saturation vapor pressure (kPa) at temperature T (C). The formula used by the LI-6400 is (14-24) on page 14-12 in the instruction manual.
Stomatal Conductance to Water Vapor
The stomatal conductance gsw to water vapor (mol H2O m-2 s-1) is obtained from the total conductance by removing the contribution from the boundary layer.
where kf is a factor based on the estimate K of the fraction of stomatal conductances of one side of the leaf to the other (termed stomatal ratio throughout this manual),
and gbw is the boundary layer conductance to water vapor (mol H2O m-2 s-1) from one side of the leaf. The boundary layer conductance correction thus depends on whether the leaf has stomata on one or both sides of the leaf.
Net photosynthesis
The mass balance of CO2 in an open system is given by
where a is assimilation rate (mol CO2 m-2 s-1), ce and co are entering and outgoing mole fractions (mol CO2 mol air-1) of carbon dioxide. Using equation 1‑2, we can write
1‑12
which rearranges to
To write equation 1‑13 in terms of what the LI-6400 measures, we use equation 1‑5 and
1‑14
where Cr and Cs are sample and reference CO2 concentrations (μmol CO2 (mol air)-1), and A is net assimilation rate of CO2 by the leaf (μmol CO2 m-2 s-1). Substitution yields
Equation 1‑15 was used by the LI-6400 until version 6.0. To get to the new equation, we substitute for E from equation 1‑6 to get
1‑16
which reduces to
Why does transpiration (or Wr and Ws in equation 1‑17) appear in the equation for photosynthesis? (Asking this question means you didn’t follow the derivation…) The short answer is that it serves as a dilution correction; as the leaf adds water vapor to the chamber, it dilutes all other gasses, including CO2.
Intercellular CO2
The intercellular CO2 concentration Ci (μmol CO2 mol air-1) is given by
where gtc is the total conductance to CO2, and is given by
1‑19
1.6 is the ratio of the diffusivities of CO2 and water in air, and 1.37 is the same ratio in the boundary layer.
Everything else
There are many other relationships that the LI-6400 uses (calibration equations for sensors, dew point temperatures, relative humidity, etc.), which are documented in Chapter 14 of the instruction manual
Summary of Symbols
- a = net assimilation rate, mol CO2 m-2 s-1
- A = net assimilation rate, μmol CO2 m-2 s-1
- ce = incoming CO2 concentration, mol CO2 mol air-1
- co = outgoing CO2 concentration, mol CO2 mol air-1
- Cs = mole fraction of CO2 in the sample IRGA, μmol CO2 mol-1 air
- Cr = mole fraction of CO2 in the reference IRGA, μmol CO2 mol-1 air
- Ci = intercellular CO2 concentration, μmol CO2 mol air-1
- E = transpiration, mol H2O m-2 s-1
- F = molar flow rate of air entering the leaf chamber, μmol s-1
- gbw = boundary layer conductance to water vapor, mol H2O m-2 s-1
- gsw = stomatal conductance to water vapor, mol H2O m-2 s-1
- gtc = total conductance to CO2, mol CO2 m-2 s-1
- gtw = total conductance to water vapor, mol H2O m-2 s-1
- kf = (K2 + 1)/(K + 1)2
- K = stomatal ratio (dimensionless); estimate of the ratio of stomatal conductances of one side of the leaf to the other
- s = leaf area, m2
- S = leaf area, cm2
- ue = incoming flow rate, mol air s-1
- uo = outgoing flow rate, mol air s-1
- we = incoming H2O mole fraction, mol H2O mol air-1
- wo = outgoing H2O mole fraction, mol H2O mol air-1
- Ws = sample IRGA mole fraction of water vapor, mmol H2O mol air-1
- Wr = reference IRGA mole fraction of water vapor, mmol H2O mol air-1
- Wl = mole fraction of water vapor within the leaf, mmol H2O mol air-1
