Colorimetric and Fluorescent Gel Staining
Protein stains are used to analyze electrophoretically separated proteins while the proteins are still in the gel. The most widely used method of electrophoresis is SDS-PAGE. Before SDS-PAGE, protein samples are denatured using an ionic detergent, such as SDS, and heat. Reducing agents, such as beta-mercaptoethanol, are often used to eliminate disulfide bonds in the proteins. Then the denatured and reduced lysate is run through a polyacrylamide gel to separate the proteins by size using electrophoresis.
During electrophoresis, an electrical current is applied to migrate the negatively charged proteins through the gel. Because smaller proteins travel through the gel more easily than larger proteins, smaller proteins will run further through the gel. A wide variety of fluorescent and colorimetric stains can be used to detect the proteins after electrophoresis.
Typical Protein Gel Imaging Workflow
Add tracking dye to lysate
Load protein samples and marker in vertical SDS-PAGE system
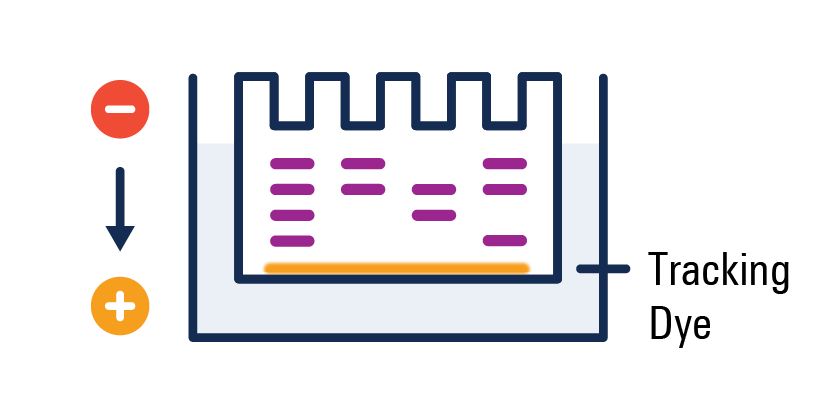
Negatively charged proteins migrate through the gel

Smaller proteins migrate further than larger proteins
SDS-PAGE gel after Coomassie blue staining

Acquire image using an Odyssey Imager
Fluorescent Staining
Fluorescent stains can be imaged in both near-infrared (NIR) and visible fluorescent wavelengths. Fluorescent staining is generally considered to be more sensitive than colorimetric staining. There are many fluorescent stains that can be imaged on Odyssey® Imagers, including:
Colorimetric Staining
Colorimetric stains, such as Coomassie blue or silver stains, can be imaged using the Odyssey M Imager. Silver stain offers more sensitivity than Coomassie, but the protocol can be complex. Coomassie stains, in contrast, are a simpler, quicker, and more affordable way to detect proteins. There are many colorimetric stains that can be detected on an Odyssey M Imager, including:
Coomassie Staining
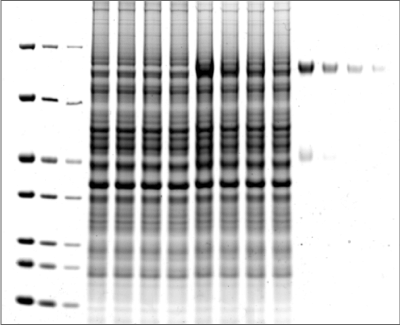
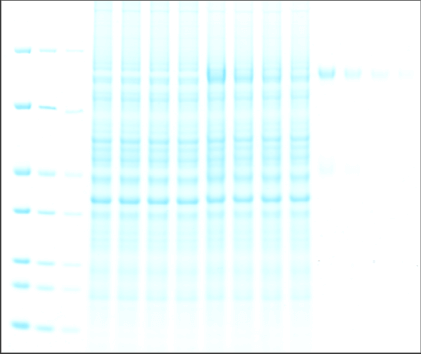
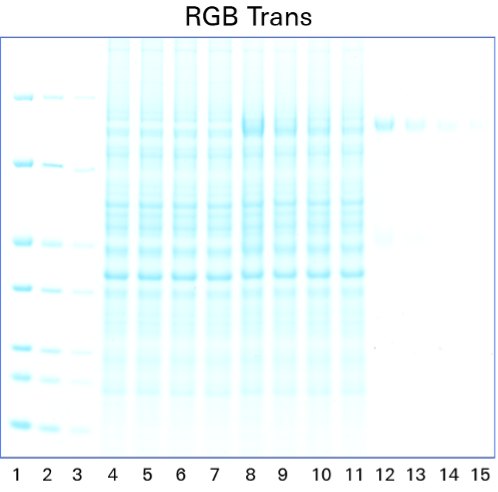
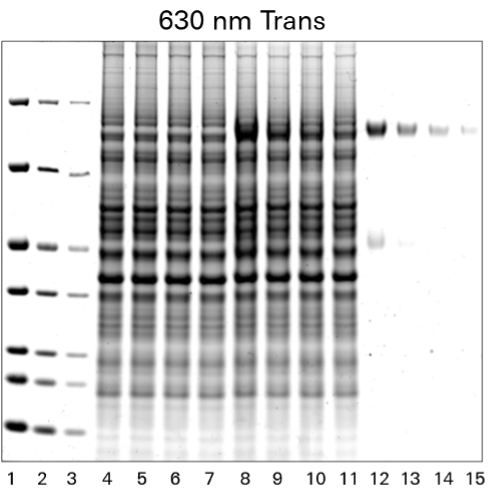
One of the most popular methods to stain a protein gel is with Coomassie blue. Coomassie blue protein stains are anionic and bind non-specifically to proteins. Coomassie blue protein stains can be used for sensitive, quantitative protein detection in gels, with a linear range from ~10 ng to 20 µg.1
Coomassie blue protein stain is a strong 700 nm fluorophore. Fluorescence of Coomassie stain is induced upon protein binding. Coomassie-stained protein gels can be imaged on any of the Odyssey Imagers using NIR fluorescence with comparable sensitivity to SYPRO™ Ruby, but at a lower cost.1
As shown in Figure 1, the Odyssey M Imager allows you to image Coomassie blue protein gels in many channels, including fluorescent and colorimetric channels.
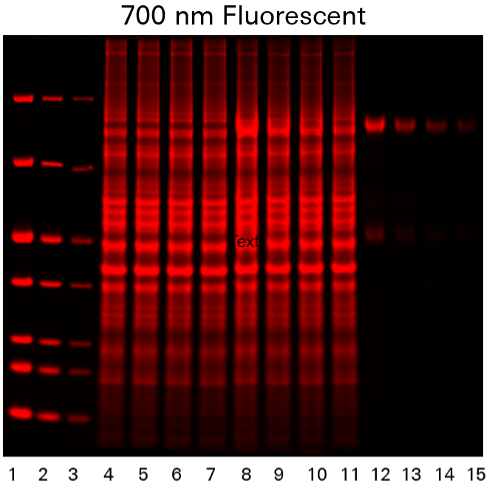
Silver Staining
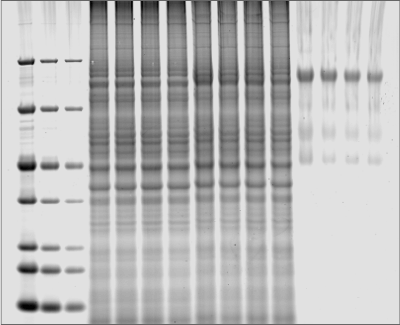
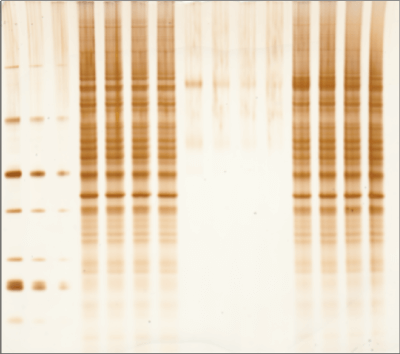
Silver staining is another popular method to identify proteins within gels after electrophoretic separation. Silver staining is a highly sensitive method of colorimetric detection that works by incubating a gel in silver nitrate to bind silver particles to proteins. The silver particles stain the proteins various shades from yellow to brown to black, which can then be imaged using the RGB Trans or 470 nm Trans channels of the Odyssey M Imager, as shown in Figure 2.
While silver staining offers high sensitivity, the protocol used can be complex and may generate high background.
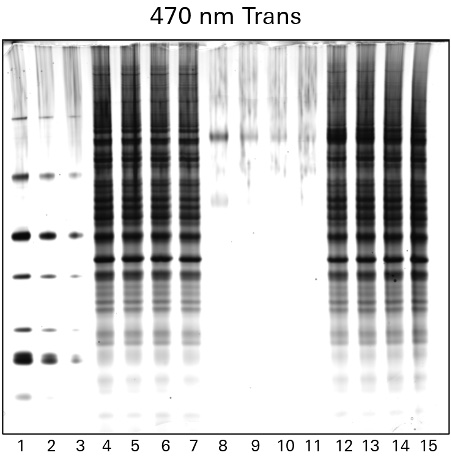
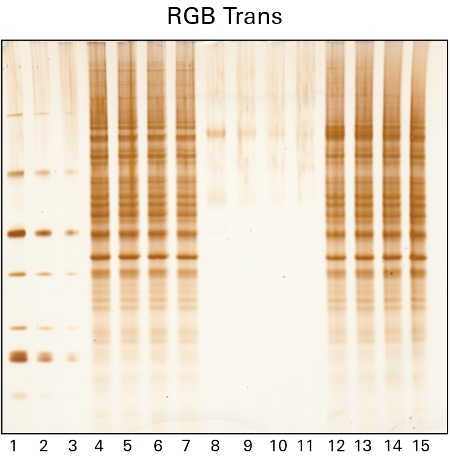
Uses for Protein Gel Staining
Protein gels are often used to assess protein purity and to detect products in forced degradation studies for the development of therapeutics. A protein gel can also be used to detect glycoproteins and phosphoproteins or can be run separately to supplement the findings of an immunoblot.
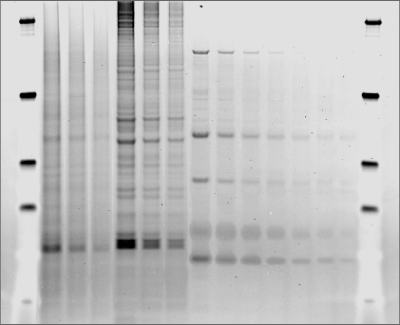
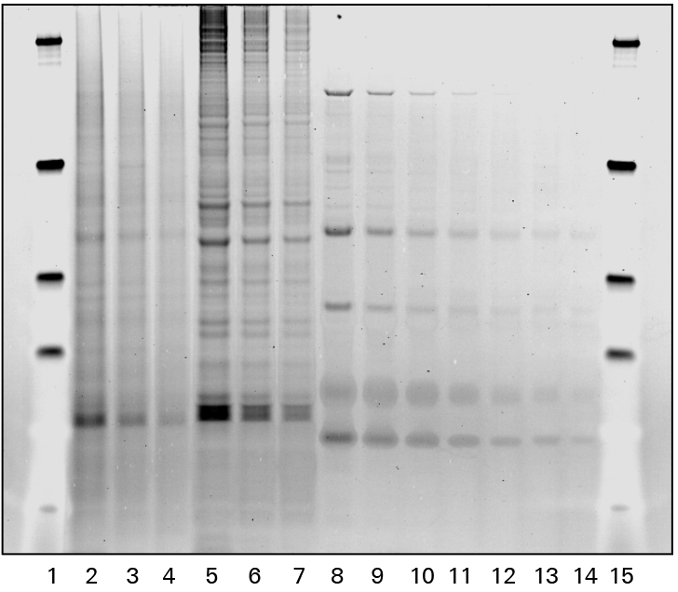
Glycoprotein Detection
Visualization of all protein bands in the gel is possible with Coomassie staining, or glycoproteins may be imaged using a glycoprotein-specific stain, such as Pro-Q™ Emerald. Glycoprotein bands with a known molecular weight can be identified by referencing a molecular weight marker lane on the gel or membrane. Shift changes due to digestions can be documented when comparing treated to non-treated samples, as shown in Figure 3.
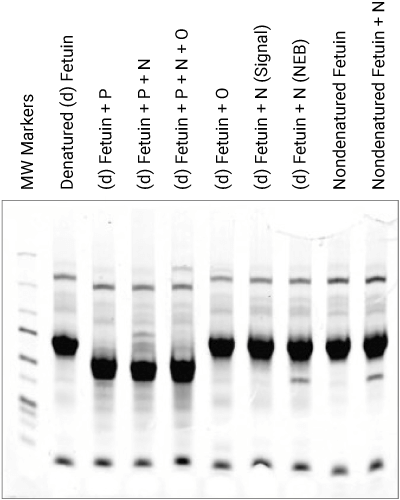
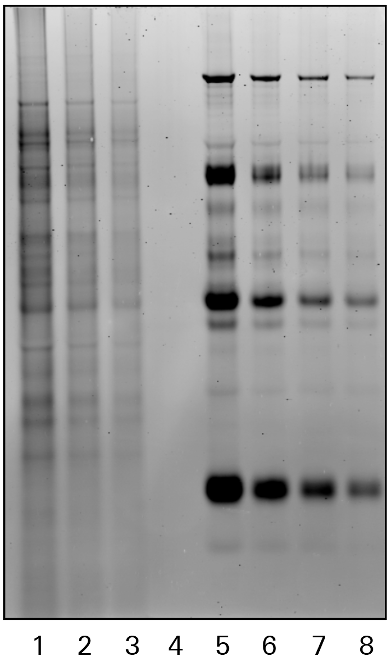
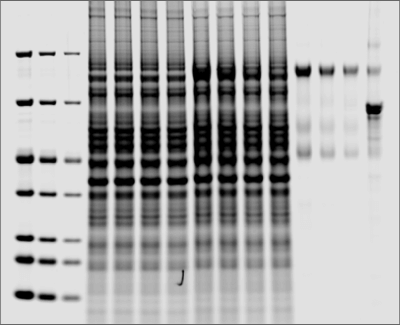
Phosphoprotein Detection
Phosphorylated protein bands may be detected within a gel without requiring transfer to a blotting membrane using Pro-Q™ Diamond Phosphoprotein Gel Stain or other phosphoprotein stains.
References
- Luo, S., Wehr, N.B., and Levine, R.L. (2006). Quantitation of protein on gels and blots by infrared fluorescence of Coomassie blue and Fast Green. Anal. Biochem., 350(2), pp. 233-238. DOI: 10.1016/j.ab.2005.10.048