On-Cell Western Assay
What It Is
An On-Cell Western (OCW) Assay is a cell-based assay that enables quantitative monitoring of cell surface protein expression. It can analyze multiple samples quickly and quantitatively, and it eliminates the use of radioactivity for the assay. The OCW Assay can be used to:
- Detect and quantify target proteins localized to the cell surface
- Quantify ligand binding to cell surface receptors
- Monitor receptor internalization and recycling by detecting loss and reappearance at the cell surface
- Perform and detect cell surface biotinylation assays
- Evaluate the effects of mutations, targeted therapeutics, and other treatments on protein expression and trafficking
How It Works
With both standard In-Cell Western™ and OCW Assays, cells are first seeded in a microplate.
With In-Cell Western Assays, cell membranes are permeabilized after cells are treated and fixed, so antibodies can reach antigens inside the cell. (Not familiar with the In-Cell Western Assay? Learn more here.) However, the permeabilization process can compromise the detection of antigens present on the cell membrane.
With OCW Assays, cells are not permeabilized because the protein targets are detected on the cell surface—not intracellularly. Instead, they are stained with antibodies against extracellular protein domains, so only cell surface antigens are detected.
The cells in both assays can then be imaged either live or post-fixation.
Ways to Use It
Monitor Receptor Internalization and Recycling
A study by Wager Miller used the OCW Assay to study the internalization and recycling of cannabinoid receptor 1 (CB1), a G protein-coupled receptor, after treatment with receptor agonists and cycloheximide. The antibodies were targeted against specific extracellular or intracellular domains of CB1. The observed time course for receptor internalization was consistent with previous confocal microscopy studies.
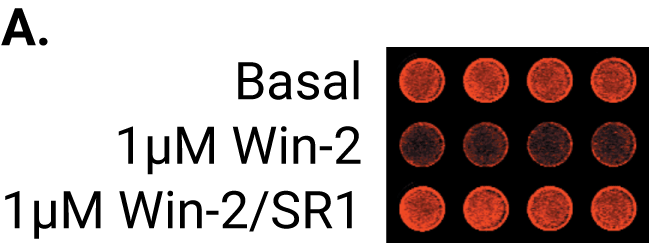
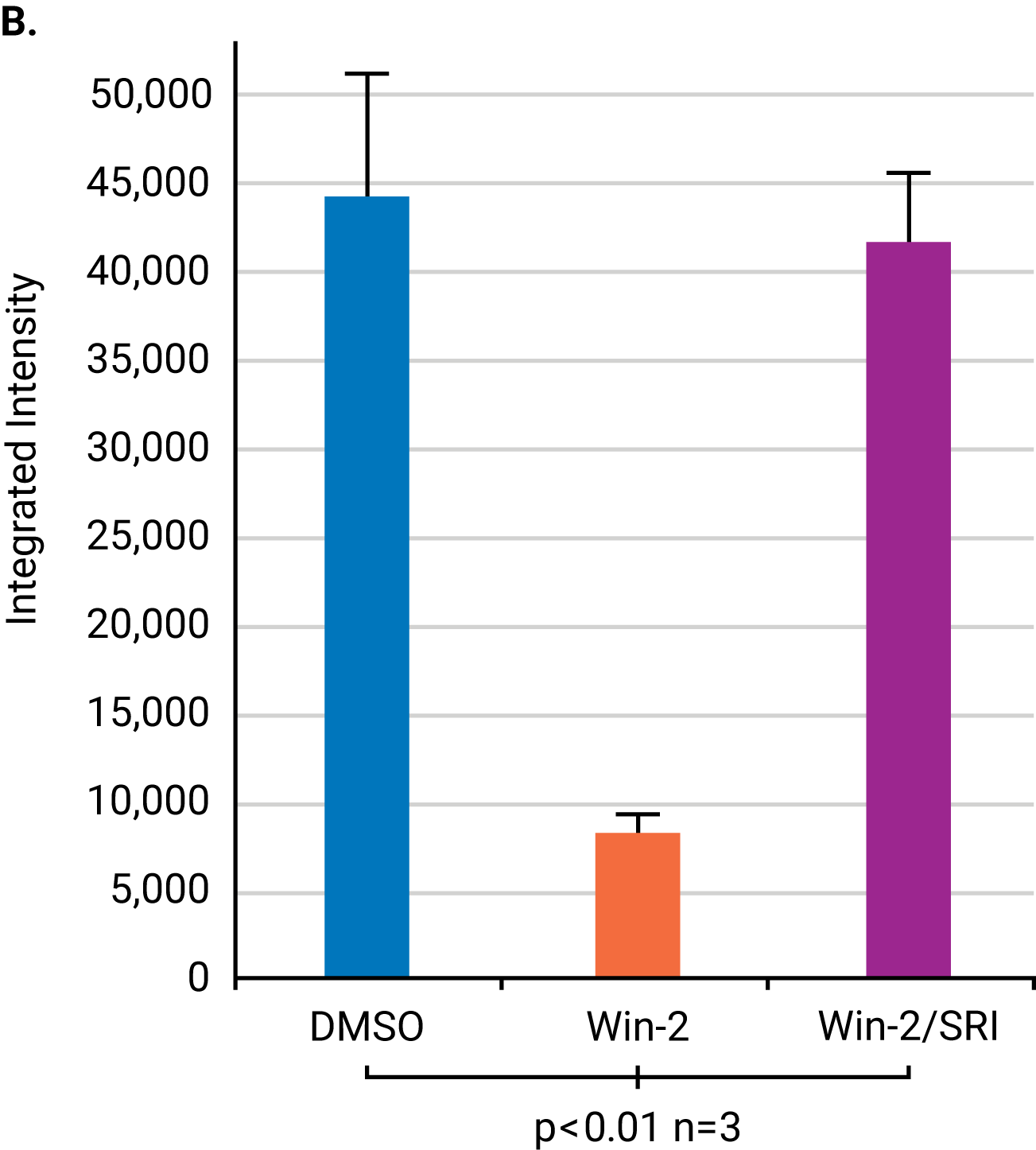
Quantify Plasmalemmal Expression of an Ion Channel
A study by Royal, Tinker, & Harmer investigated the roles of two phosphatidylinositols—Phosphatidylinositol-4,5-bisphosphate (PIP2) and Phosphatidylinositol-4-phosphate (PI(4)P)—in the anterograde trafficking of KCNQ channels. They used OCW and ICW Assays to determine the surface expression (Fig. 2A) and trafficking (Fig. 2B) of VSV-KCNE1-KCNQ1 (VSV-E1-Q1), a construct whose VSV epitope was used to monitor cell-surface expression of the KCNQ1-KCNE1 (Q1/E1) channel complex.
Overall, results indicate that trafficking and expression are not affected by PIP2 and/or PI(4)P reduction at the plasma membrane or by PI(4)P reduction at the Golgi. Consequently, the Q1/E1 channel may not require PIP2 or PI(4)P for anterograde trafficking. However, PIP2 may be necessary for the activation of the Q1/E1 channel once at the plasma membrane (PM).
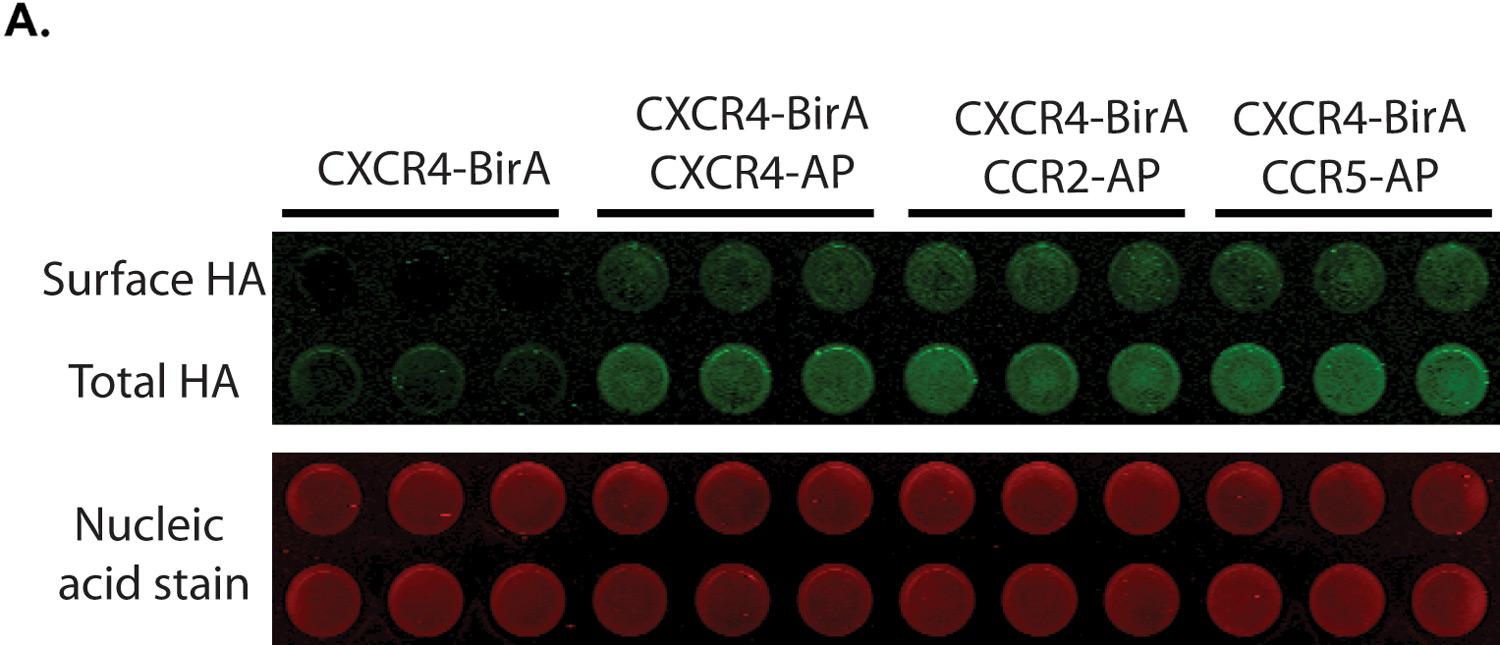
Confirm Membrane Expression of GPCR Constructs
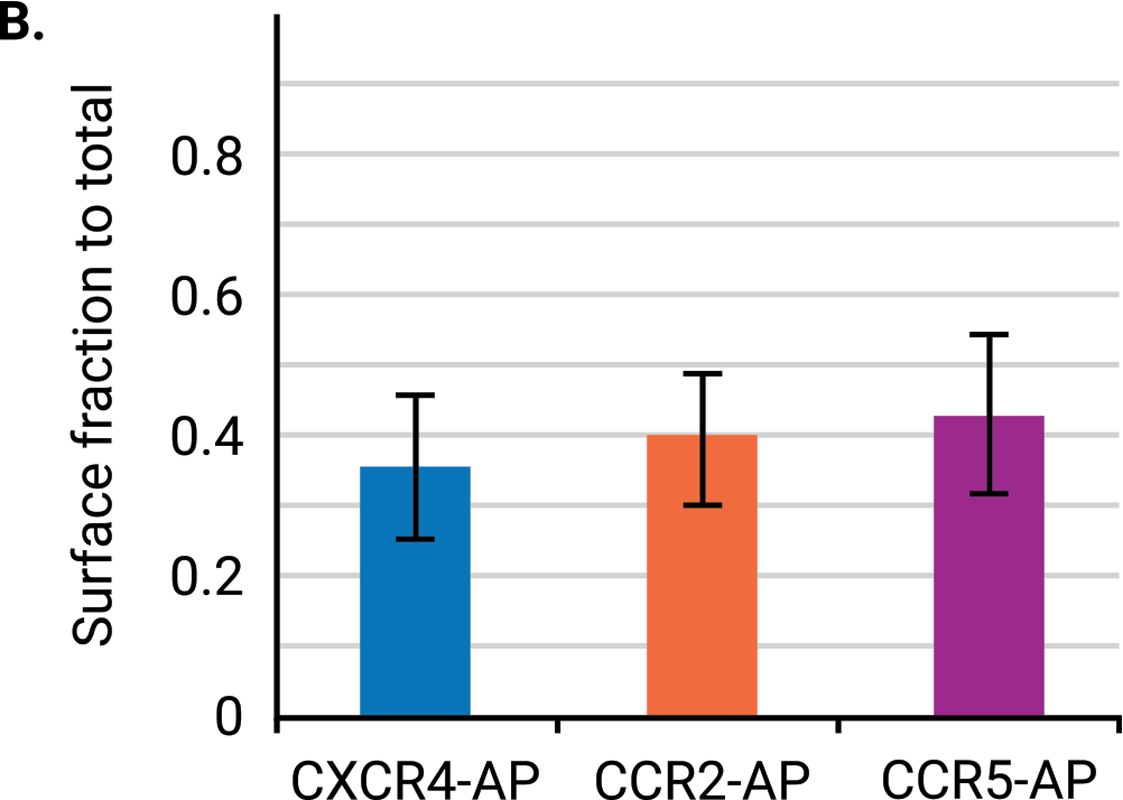
A study by Steel, Murray, and Liu examined the dimerization of G protein-coupled receptors (GPCRs), which help mediate human physiological responses by engaging with signaling pathways. To test a multiplex biotinylation assay, they used three chemokine receptors: CXCR4, CCR2, and CCR5. An OCW Assay was used to confirmed surface expression of the HA-tagged receptors (Fig 3). The proximity-based biotinylation assay confirmed CXCR4 homodimerization and CXCR4-CCR2 and CXCR4-CCR5 heterodimerization.
Resources
- On-Cell Western Plate-Based Assay for Targeted Near-Infrared-Labeled Optical Imaging Agent Development: Receptor-Based Binding and Competition Assays Application Guide
- Labeling of Fixed Cells with IRDye NHS Ester Reactive Dyes for In-Cell Western Assay Normalization Application Guide
- A cell-based immunocytochemical assay for monitoring kinase signaling pathways and drug efficacy white paper
- Tracking G protein-coupled receptor trafficking using Odyssey imaging
- Purification method directly influences effectiveness of an epidermal growth factor-coupled targeting agent for noninvasive tumor detection in mice.
Application Guides
White Papers
See published examples of On-Cell Western Assays
View publicationsReferences
- Wager-Miller, J. (2004). Tracking G Protein-coupled Receptor Trafficking Using Odyssey Imaging.
- Royal, A.A., Tinker, A., & Harmer, S.C. (2017). Phosphatidylinositol-4,5-Bisphosphate Is Required for KCNQ1/KCNE1 Channel Function but Not Anterograde Trafficking. PLoS ONE 12(10): e0186293. https://doi.org/10.1371/journal.pone.0186293
- Steel, E., Murray, V.L., & Liu, A.P. (2014). Multiplex Detection Of Homo- And Heterodimerization Of G Protein-Coupled Receptors By Proximity Biotinylation. PLoS ONE 9(4): e93646. https://doi.org/10.1371/journal.pone.0093646
