Regulatory Products and Services
LICORbio offers an extensive range of products and services designed to help with your regulated laboratory's needs. Regulatory solutions supplement our imaging systems, software, reagents, and other services.
21 CFR Part 11-Ready Software
Image Studio™ 21 CFR Part 11 software is available to help your lab comply with the Food and Drug Administration’s (FDA) 21 CFR Part 11 regulations. Regulated labs with a controlled workflow can ensure traceability for access control, data acquisition, and analysis.
A trained LICORbio technician will install Image Studio 21 CFR Part 11 software according to LICORbio standard operating procedures. They will also work with your IT personnel to integrate the software into your existing IT infrastructure and provide training for administration of the software.
For more details on how Image Studio 21 CFR Part 11 addresses needs of regulated laboratories, read the compliance guide.Image Studio 21 CFR Part 11 Features
- Image archive
- Electronic signature and approval
- Time and date stamped change logs
- Version control
- System and record accessibility restriction

Services
The following is an overview of available services. Contact the regulatory service experts at regulatory@licor.com for more information.
Installation Qualification (IQ)
A certified LICORbio technician will verify correct instrument installation and complete the appropriate documentation to demonstrate that the system has been installed according to LICORbio standards.
Recommended for labs that are audited externally.
Operational Qualification (OQ)
A certified LICORbio technician will verify that your instruments are performing according to LICORbio standards. No additional charges will be incurred for a Manufacturer Audit.
Recommended for labs that are audited externally.

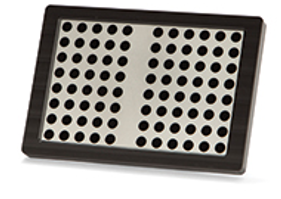
Odyssey® Imager Verification Plates
Odyssey verification plates are used to verify consistent performance of your Odyssey Imager over time. Verification plates are used as part of OQ procedures to ensure that the Odyssey Imager has not encountered problems that would interfere with consistent imaging and quantification. Purchasing your own verification plates makes it easier to assess your Odyssey Imager regularly and enables you to use the plate in your own testing protocols (for example, testing your Odyssey Imager between batches).
Manufacturer Audit (MA)
Verify that LICORbio is performing up to your standards. Visit our headquarters in Lincoln, Nebraska, USA and complete an on-site audit as required by regulatory agencies or your organizational standard operating procedures. LICORbio hosts auditors and provides necessary information for auditors and/or regulatory officials.