IRDye® Peptide Labeling
Introduction
Peptides labeled with fluorescent dyes are important as probes for in vivo imaging and as substrates for enzyme activity assays. Near-infrared (NIR) fluorophores such as IRDye® 800CW (excitationMax 774 nm; emissionMax 789 nm) can offer improved sensitivity because of low NIR autofluorescence from tissues, cells, biological materials, or drug compounds.
Fluorophores can also be combined with appropriate quencher dyes to create fluorogenic peptide probes, such as those used for protease activity detection (1 – 4). IRDye QC-1 can efficiently quench a wide range of fluorophores spanning the visible and NIR spectra (~500–800 nm) in a fluorescence resonance energy transfer (FRET) system. Together, IRDye 800CW and IRDye QC-1 comprise an optimal fluorophore-quencher pair for incorporation into NIR fluorogenic peptides (Figure 167).
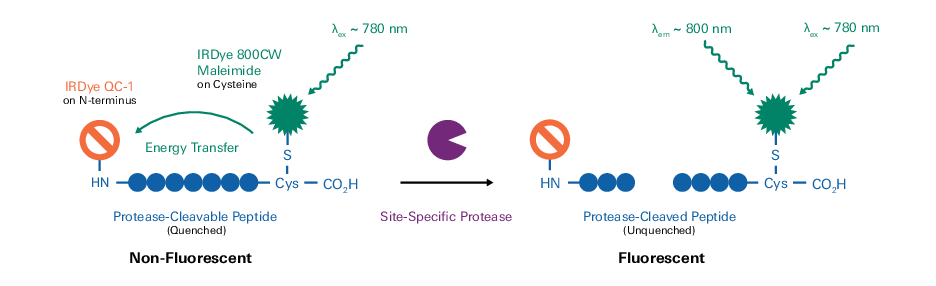
IRDye fluorophores and quenchers are amenable to a wide variety of conjugation reactions that yield stable, covalently-labeled peptides. Furthermore, site-specific dye labeling can be achieved by employing orthogonally reactive groups in peptide design. Because IRDye labels have been optimized for detection in aqueous biological environments, conjugation reactions are best performed in aqueous-phase.
Peptide Labeling Options
IRDye NHS esters react with unprotected amines in peptides. NHS ester reactions usually proceed quickly and cleanly in either organic or aqueous solvents. While NHS esters continue to be widely used tools for biomolecule modification, their application to large peptides may be complicated by factors, such as steric hindrance and multiple labeling sites.
To address the limitations of NHS ester chemistry, orthogonal technologies such as IRDye Maleimides and Click Chemistry Reagents have been developed to label non-amine functional groups. Orthogonal chemistry can also facilitate dual- or multi-dye labeling. As with NHS ester chemistry, optimal non-amine labeling is usually achieved by aqueous solution-phase reactions. Table 23 summarizes general strategies for labeling peptides with IRDye reagents.
IRDye Maleimides are designed to react selectively with sulfhydryl groups in the presence of amines. Optimal selectivity is usually obtained with labeling reactions performed in mildly acidic (pH 6.5) aqueous solvents. At acidic pH, amine reactivity is greatly reduced because they are prevalently protonated. Sulfhydryl groups can be installed in peptides with cysteine or commercially available thiol-linkers.
Peptides containing sulfhydryls can spontaneously form inactive dimers or cyclize internally. Therefore, it may be necessary to pre-treat the peptide with a reducing agent such as tris(2-carboxyethyl)- phosphine (TCEP) to reveal reactive sulfhydryl functional groups.
IRDye Click Chemistry reagents are mostly inert to naturally occurring functional groups such as amines and hydroxyls. Click Chemistry utilizes pairs of functional groups that can react either in the presence (5) or absence (6) of a copper catalyst. Click Chemistry functional groups can also be readily installed in peptides with commercially available reagents.
Labeling Reaction Techniques
Aqueous Solution-Phase Labeling
For all labeling reactions, it is critical to omit components that can interfere with the coupling of the peptide and the dye. Peptides should be dissolved in aqueous buffers that do not contain extraneous nucleophiles such as Tris, sodium azide, DTT, BME, etc.
To maximize the labeling efficiency, the peptide should be dissolved at the highest practical concentration that still maintains a homogeneous reaction mixture. The aqueous reaction mixture can be directly purified by reverse-phase HPLC (see Purification). Table 24 provides general parameters for aqueous solution-phase labeling of peptides with IRDye reagents.
* Copper-catalyzed Click Chemistry (5)
† Copper-free Click Chemistry (6)
Organic Solution-Phase Labeling
Certain peptides, such as those comprised mainly of hydrophobic amino acids, may not be amenable to aqueous solution-phase labeling. In these atypical cases, IRDye reagents can be used for organic solution-phase labeling; however, this reaction may be slower than its aqueous counterpart.
Again, peptides should be dissolved in solvents devoid of extraneous nucleophiles such as Tris, sodium azide, DTT, BME, etc. The optimal reaction solvent is anhydrous dimethyl sulfoxide (DMSO). When labeling with IRDye NHS esters and Maleimides, including a tertiary amine base such as N,N-diisopropylethylamine (DIPEA) is necessary to promote the reaction. After the reaction has completed, the crude IRDye labeled peptide can be precipitated from the DMSO solution by dropwise addition into anhydrous diethyl ether.
IRDye labels impart hydrophilicity and can facilitate the purification of hydrophobic peptides by reverse-phase HPLC (see Purification). Table 25 provides general parameters for organic solution-phase labeling of peptides with IRDye reagents.
* Copper-Catalyzed Click Chemistry (5)
† Copper-Free Click Chemistry (6)
Purification
Reverse-phase HPLC purification delivers IRDye labeled peptides with the highest purity. The recommended mobile phase is a gradient mixture of acetonitrile and water buffered with triethylammonium acetate (TEAA, 50 mM, pH 6.0). The TEAA provides ion-pairing for better retention behavior of the hydrophilic IRDye labeled peptides on the column.
As a consequence, the predominant counter-ion of the purified peptide will be triethylammonium, which may interfere with downstream biological experiments. Prior to lyophilization, the purified peptide should be ion-exchanged by eluting through an appropriate resin (e.g., Amberlite™) or by dialysis.
For flexibility in development and/or troubleshooting, the HPLC system should be equipped with a diode array detector (DAD) and be able to monitor at the absorption maxima of all dyes used.
Quantification
UV-Vis absorbance spectroscopy is the best method for quantifying the amount of purified IRDye labeled peptide. To determine the concentration of a stock solution containing a singly-labeled peptide, dilute an aliquot of the stock solution in 1X PBS, measure the absorbance spectra at the dye-specific maximum and use the following equation:
In which:
AIRDye is the measured absorbance at the dye-specific maximum (See Table 26)
εIRDye is the extinction coefficient for the dye in 1X PBS (See Table 26)
Dilution Factor is the fold dilution of the IRDye peptide in 1X PBS
MWIRDye peptide is the molecular weight of the IRDye peptide
For a pure stock solution of a FRET-quenched peptide labeled with exactly one IRDye 800CW and one IRDye QC-1: Determine the concentration of a stock solution by diluting an aliquot of the stock solution in methanol, measuring the absorbance spectra at 778 nm and 850 nm and using the following equation:
In which:
A778 is the measured absorbance at the maximum for IRDye 800CW in methanol
300,000 is the extinction coefficient for IRDye 800CW in methanol
1.265 is the correction factor for the ratio of A778/A850 for IRDye QC-1 in methanol
A850 is the measured absorbance at the maximum for IRDye QC-1 in methanol
Dilution Factor is the fold dilution of the IRDye 800CW QC-1-peptide in methanol
MWIRDye 800CW QC-1 peptide is the molecular weight of the IRDye 800CW QC-1 peptide
Examples
For an example of aqueous solution-phase labeling with IRDye 800CW NHS ester, see Davies-Venn, C.A., Angermiller, B., Wilganowski, N., Ghosh, P., Harvey, B.R., Wu, G., et al. (2012). Albumin-Binding Domain Conjugate for Near-Infrared Fluorescence Lymphatic Imaging. Mol. Imaging Biol, 14(3), 301–14. DOI: 10.1007/s11307-011-0499-x
For an example of organic solution-phase labeling with IRDye 800CW NHS ester, see Chen, Y., Dhara, S., Banerjee, S.R., Byun, Y., Pullambhatla, M., Mease, R.C., and Pomper, M. (2009). A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem. Biophys. Res. Commun, 390(3), 624–9. DOI: 10.1016/j.bbrc.2009.10.017
For an example of aqueous solution-phase labeling with IRDye 800CW Maleimide, see Ye, Y., Zhu, L., Ma, Y., Niu, G., and Chen, X. (2011). Synthesis and evaluation of new iRGD peptide analogs for tumor optical imaging. Bioorg. Med. Chem. Lett, 21(4), 1146–50. DOI: 10.1016/j.bmcl.2010.12.112
For examples of dual-labeling with IRDye QC-1 NHS ester and various fluorophores, see Sun, X., Zhang, A., Baker, B., Sun, L., Howard, A., Buswell, J., et al. (2011). Development of SNAP-Tag Fluorogenic Probes for Wash-Free Fluorescence Imaging. ChemBioChem, 12(14), 2217–26. DOI: 10.1002/cbic.201100173
References
1. Matayoshi, E.D., Wang, G.T., Krafft, G.A., and Erickson, J. (1990). Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science, 247(4945), 954–8. DOI: 10.1126/science.2106161
2. Bullok, K.E., Maxwell, D., Kesarwala, A.H., Gammon, S., Prior, J.L., Snow, M., et al. (2007). Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochem., 46(13), 4055–65. DOI: 10.1021/bi061959n
3. Zheng, G., Chen, J., Stefflova, K., Jarvi, M., Li, H., and Wilson, B.C. (2007). Photodynamic molecular beacon as an activatable photosensitizer based on protease controlled singlet oxygen quenching and activation. Proc. Natl. Acad. Sci. USA, 104 (21), 8989–94. DOI: 10.1073/pnas.0611142104
4. Blum, G., Mullins, S.R., Keren, K., Fonovic, M., Jedeszko, C., Rice, M.J., et al. (2005). Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nature Chem. Biol., 1(4), 203–9. DOI: 10.1038/nchembio728
5. Best, M.D. (2009). Click Chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry, 48(28), 6571–84. DOI: 10.1021/bi9007726
6. Simon, M., Zangemeister-Wittke, U., and Plückthun, A. (2012). Facile double-functionalization of designed ankyrin repeat proteins using Click and thiol chemistries. Bioconjugate Chem., 23(2), 279–86. DOI:10.1021/bc200591x