Blocking Buffer Optimization Protocol
Introduction
This document describes a method to determine optimal blocking conditions for NIR Western blot detection with the Odyssey family of imagers. The specific lysate and antibodies used in your system will be evaluated in four different blocking buffer solutions. The buffering system will also be evaluated.
Choosing Blocker and Buffer System
The experimental design suggested in this document is set up to let you compare three specific blocking buffers to a blocking buffer and buffer system of your choice.
At this point, you can choose the blocking buffer that you would like to test. When choosing a blocking buffer, it is best to keep these considerations in mind:
- TBS-based blocking buffers are generally used to detect phospho-proteins, because the phosphate present in PBS blocking buffers may competitively bind with antibodies to phospho-proteins.
- Intercept® Blocking Buffer (licor.com/intercept) is available in protein-based and protein-free formulations in TBS and PBS.
- Be sure to keep your buffer system consistent throughout the protocol for blocking, antibody dilutions, and washes. For example, if you use a TBS-based buffer system, choose Intercept® (TBS) Blocking Buffer. If you use a PBS-based buffer system, choose Intercept® (PBS) Blocking Buffer.
Required Reagents
Odyssey Protein Molecular Weight Marker (928-40000)
IRDye® Secondary Antibodies
Intercept (TBS) Blocking Buffer (927-60001)
Intercept (PBS) Blocking Buffer (927-70001)
Intercept (TBS) Protein-Free Blocking Buffer (927-80001)
Blocking buffer of your choice (milk, BSA, etc.)
Membrane: Odyssey Nitrocellulose (926-31090, 926-31092) or PVDF Membrane Kit with 4X Protein Loading Buffer (926-31097)
Primary antibodies
Primary antibodies must be from host species compatible with the secondary antibodies being used. If you are using subclass specific antibodies, please refer to this technical note: Western Blot and In‑Cell Western™ Assay Detection Using IRDye Subclass Specific Antibodies (licor.com/subclass).
Tween® 20
PBS Buffer (1X)
TBS Buffer (1X)
Methanol (when using Immobilon®-FL PVDF membrane)
SDS (when using Immobilon-FL PVDF membrane)
Western Blot Incubation Box (929-97201, 929-97205, or 929-97210)
Odyssey Family Imager
Handling Gels
Standard protein electrophoresis conditions and reagents can be used for gel and sample preparation.
Handling Membranes
- Western blots should be prepared using standard blotting procedures and Millipore Immobilon-FL PVDF or Odyssey Nitrocellulose Membrane.
- Only handle membranes by the edges with clean forceps. Be careful not to touch the membrane with your hands or gloves.
- Do not write on membranes with regular ink pens or markers, because the ink will fluoresce on Odyssey Imaging Systems. You can write on nitrocellulose membranes with pencil or the Odyssey Pen (PN 926-71804). Use only a pencil to write on PVDF membranes, because the ink from the Odyssey Pen will dissolve in the methanol used to wet the PVDF membrane.
Protocol
Step 1. Load Gel
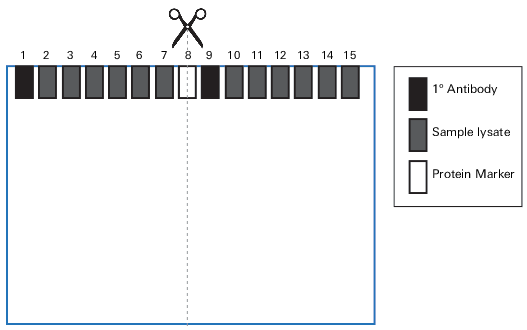
Following is a suggested template for loading a gel for the blocker optimization experiment.
You will be loading a serial dilution in duplicate and in random sample order. Using two 15-well gels, load the following samples in the order indicated in the table below.
The actual amounts you load may vary depending on the particular conditions in your experiment. For example, you may need to use a different starting value for your dilution series to ensure that your target can be detected in every dilution.
Lane | Sample | Amount |
1 | Primary Antibody (as positive control) | 5 ‑ 10 ng |
2 | Sample Lysate | 313 ng |
3 | Sample Lysate | 625 ng |
4 | Sample Lysate | 1.25 µg |
5 | Sample Lysate | 2.5 µg |
6 | Sample Lysate | 5 µg |
7 | Sample Lysate | 10 µg |
8 | Protein Marker | 1-3 µL |
9 | Primary Antibody (as positive control) | 5 ‑ 10 ng |
10 | Sample Lysate | 313 ng |
11 | Sample Lysate | 625 ng |
12 | Sample Lysate | 1.25 µg |
13 | Sample Lysate | 2.5 µg |
14 | Sample Lysate | 5 µg |
15 | Sample Lysate | 10 µg |
Run the gel according to the manufacturer's instructions.
Step 2. Transfer to Membrane
Transfer to nitrocellulose or PVDF membrane.
More information about transferring proteins to a membrane is available here licor.com/proteintransfer.
Step 3. Dry Membrane
Allow blots to dry before proceeding with detection. Blots can be dried using any of the following options.
- Place the membrane on a piece of dry filter paper. Leave the membrane and filter paper on the benchtop for about an hour.
- Place the membrane on a piece of dry filter paper. Leave the membrane and filter paper in a 37 °C oven for about 10 minutes.
- Place the membrane between two pieces of dry filter paper, and place the membrane and filter papers into a protected place overnight. A drawer or a cabinet shelf may suffice.
Once dry, blots can be stored overnight at room temperature in a safe place between two sheets of new filter paper or using Western Blot Storage Bags (929-95100).
Step 4. Cut Membranes
If using the gel configuration described in Section III, cut each membrane through the protein marker in Lane 8 to generate four individual blots (Figure 54).
Step 5. Wet Membranes
Place cut membranes into 4 different Western Blot Incubation Boxes and wet.
Be sure to thoroughly clean each Western Blot Incubation Box with methanol prior to use.
For Immobilon®-FL PVDF membranes
Wet for 30 seconds in 100% methanol.
- Wet pieces in 1X TBS or 1X PBS (as appropriate for your experiment, see Choosing Blocker and Buffer System) for 5 minutes.
For Odyssey Nitrocellulose Membranes
Wet pieces in 1X TBS or 1X PBS (as appropriate for your experiment, see Choosing Blocker and Buffer System) for 5 minutes.
Step 6. Block Membranes
Block with 10 mL Blocking Buffer for 1 hour at room temperature with gentle shaking. Be sure to use sufficient blocking buffer to cover the membrane (a minimum of 0.4 mL/cm2 is suggested).
- Box 1: Intercept® (TBS) Blocking Buffer
- Box 2: Intercept (PBS) Blocking Buffer
- Box 3: Intercept (TBS) Protein-Free Blocking Buffer
- Box 4: Blocking buffer chosen in Choosing Blocker and Buffer System.
Step 7. Dilute Primary Antibody
Dilute primary antibody in 10 mL of appropriate diluent.
Prepare the primary antibody diluent: Add Tween® 20 to blocking buffer for a final concentration of 0.2% Tween 20. Alternatively, use Intercept T20 Antibody Diluent (preformulated with Tween 20) to avoid having to measure and dilute Tween 20 yourself.
- Box 1: Intercept (TBS) Blocking Buffer) + 0.2% Tween 20
- Box 2: Intercept (PBS) Blocking Buffer + 0.2% Tween 20
- Box 3: Intercept (TBS) Protein-Free Blocking Buffer + 0.2% Tween 20
- Box 4: Box 4: Blocking buffer chosen in Choosing Blocker and Buffer System + 0.2% Tween 20
Dilute primary antibody in antibody diluent using the vendor's recommendations.
Antibody dilution may vary depending on the primary antibody.
Step 8. Incubate Blots in Diluted Primary Antibody
Carefully pour off blocking buffer, then incubate the blot in primary antibody solution for 1 - 4 hours at room temperature or overnight at 4 °C with gentle shaking.
Optimal incubation times vary for different primary antibodies.
If the procedure cannot be completed in full, this is a good place to stop until the following day. Incubate the primary antibody overnight at 4 °C with gentle shaking.
Step 9. Wash Membranes
Carefully pour off primary antibody solution.
Wash each blot with a washing buffer that matches the buffer system used for blocking. Wash blots by shaking vigorously on platform shaker at room temperature for 5 minutes.
- Box 1: Rinse with 1X TBS-T (0.1% Tween® 20). Cover blot with 1X TBS-T for washing.
- Box 2: Rinse with 1X PBS-T (0.1% Tween 20). Cover blot with 1X PBS-T for washing.
- Box 3: Rinse with 1X TBS-T (0.1% Tween 20). Cover blot with 1X TBS-T for washing.
- Box 4: Use 1X TBS-T (0.1% Tween 20) or PBS-T (0.1% Tween 20), depending on the blocking buffer and buffer system chosen in Choosing Blocker and Buffer System.
Pour off wash solution.
Repeat 3 additional times.
Step 10. Dilute Secondary Antibody
Dilute secondary antibody in 10 mL of appropriate diluent. Intercept® T20 Antibody Diluent (preformulated with Tween 20) can be used, so you can avoid having to measure and dilute Tween 20 yourself.
For IRDye® 800CW, 680RD, and 680LT Secondary Antibodies, the recommended starting dilution is 1:20,000. See your pack insert for details.
For Immobilon®-FL PVDF membranes:
Box 1: Intercept® (TBS) Blocking Buffer + 0.2% Tween 20 + 0.01% SDS + Secondary Antibody
Box 2: Intercept (PBS) Blocking Buffer + 0.2% Tween 20 + 0.01% SDS + Secondary Antibody
Box 3: Intercept (TBS) Protein-Free Blocking Buffer + 0.2% Tween 20 + 0.01% SDS + Secondary Antibody
Box 4: Blocking buffer and buffering system chosen in Choosing Blocker and Buffer System + 0.2% Tween 20 + 0.01% SDS + Secondary Antibody
For Odyssey Nitrocellulose Membrane:
Box 1: Intercept (TBS) Blocking Buffer + 0.2% Tween 20 + Secondary Antibody
Box 2: Intercept (PBS) Blocking Buffer + 0.2% Tween 20 + Secondary Antibody
Box 3: Intercept (TBS) Protein-Free Blocking Buffer + 0.2% Tween 20 + Secondary Antibody
Box 4: Blocking buffer and buffering system chosen in Choosing Blocker and Buffer System + 0.2% Tween 20 + Secondary Antibody
Step 11. Incubate Blot in Secondary Antibody
Protect membranes from light during incubation.
Incubate blot in diluted secondary antibody for 1 hour at room temperature with gentle shaking.
Do not incubate for longer than 1 hour, because the background may increase.
Step 12. Wash Membrane
Protect membranes from light during washes.
Carefully pour off secondary antibody solution.
Wash each blot with a washing buffer that matches the buffer system used for blocking, as described in Block Membranes. Wash blots by shaking vigorously on platform shaker at room temperature for 5 minutes.
Box 1: 1X TBS-T (0.1% Tween® 20)
Box 2: 1X PBS-T (0.1% Tween 20)
Box 3: 1X TBS-T (0.1% Tween 20)
Box 4: Use 1X TBS-T (0.1% Tween 20) or PBS-T (0.1% Tween 20), depending on the blocking buffer and buffering system chosen in Choosing Blocker and Buffer System.
Pour off wash solution.
Repeat 3 additional times.
Step 13. Rinse Membrane
Rinse each membrane with 1X TBS or 1X PBS (as appropriate) to remove residual Tween 20.
Step 14. Scan Membrane
Protect membranes from light prior to scanning.
Scan the membrane on an Odyssey Imaging System.
The membrane can be scanned wet or dry. You may want to first image your membrane wet and then image it dry to determine the best conditions for imaging your blot. Scanning the membrane dry can add signal intensity, but can also lead to increased background.
Analyze Blots
With Empiria Studio® Software, you can evaluate the results of the experiment using visual inspection of your blots or quantitative blot analysis. Briefly, these Empiria Studio features are helpful for evaluating the blots from this experiment:
Adjust the image display settings for each blot to see the features on the image that you need to see.
Quantify bands and calculate Signal-to-Noise ratio for bands.
Choose the blocking conditions that are most appropriate for the context and goals of your experiment. The best blocking conditions depend on the antigen-antibody pair you are using. Look for blocking buffer conditions that provide:
Strong signals for the expected band(s)
Low membrane background
Few non-specific background bands from the primary antibody
Some primary antibodies are dramatically affected by blocking conditions. An inappropriate blocker can alter binding specificity, affecting the intensity of target bands and increasing non-specific banding. The pattern of non-specific bands may also be affected.
Blocking conditions that yield very strong bands might also have higher membrane background or non-specific banding. Because trade-offs may be necessary, it is worth taking the time to optimize your blocking buffer for your experiment.