Glycoprotein Staining
With two-color near-infrared detection, the Odyssey® Imager offers a variety of applications for glycoprotein detection with multiplexing capabilities.
Near-infrared wavelengths provide low background from biological materials, buffer components, and standard membranes used in Western blotting and lectin blotting applications. The Odyssey Imager and IRDye® dye-labeled conjugates provide a single optimized solution for detecting a variety of glycoprotein interactions.1
Glycoprotein Detection Types
- Total Protein Detection for Glycoprotein Digestions (Coomassie staining)
- Indirect Detection Using IRDye Labeled Streptavidin
- Direct Detection with an IRDye Labeled Lectin
- Glycoprotein Antibody Detection
- Enzymatic Glycoprotein Detection
Total Protein Detection for Glycoprotein Digestions (Coomassie staining)
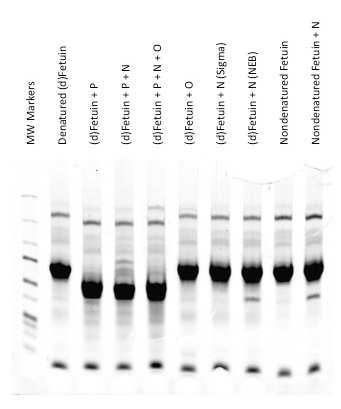
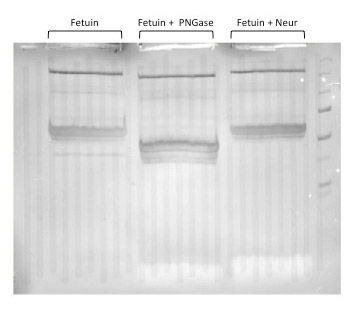
Visualization of all protein bands is possible with Coomassie staining. Glycoprotein bands with a known molecular weight can be identified by referencing a molecular weight marker lane on the gel or membrane. Phenomena such as shift changes due to denaturations or digestions can be documented when comparing treated to non-treated samples.
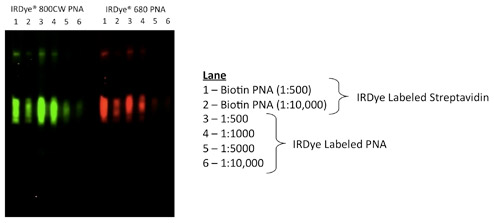
Indirect Detection using IRDye Labeled Streptavidin
Biotinylated lectins are commercially-available from several vendors and can be used in combination with IRDye Streptavidin to detect glycoproteins.
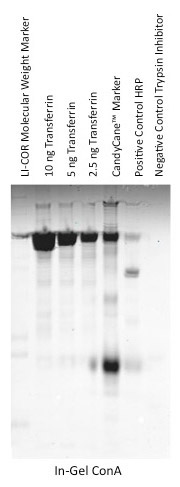
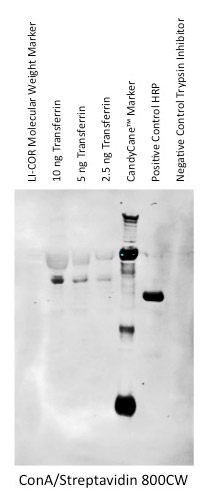
Direct Detection with an IRDye Labeled Lectin
Lectins can be covalently labeled with IRDye infrared dyes using an IRDye Protein Labeling kit. Labeled lectins can used to detect glycoproteins directly.
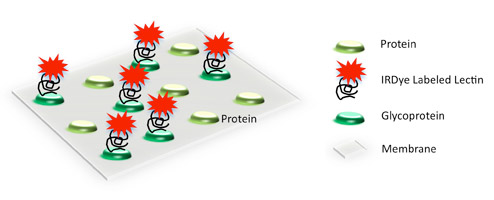
Glycoprotein Antibody Detection
O-GlcNAc (O-linked N-acetylglucosamine) modifications can be detected using a specific primary antibody and IRDye secondary antibodies.
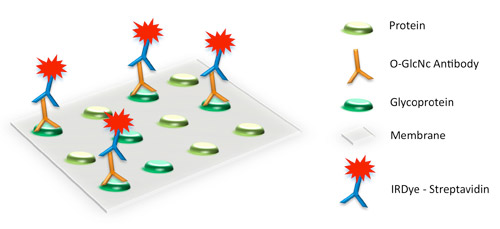

Enzymatic Glycoprotein Detection
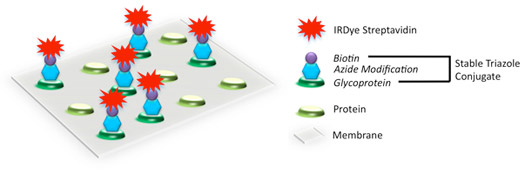
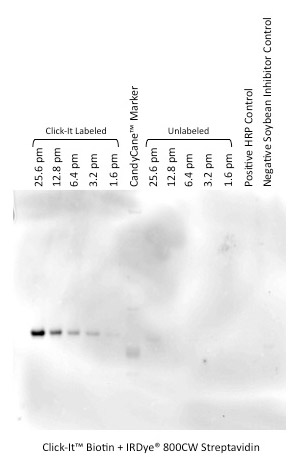
O-linked glycosylated proteins can be modified with the Click-iT™ O-GlcNAc enzymatic labeling kit and detected using the Click-IT Biotin Glycoprotein Detection Kit in combination with IRDye 800CW Streptavidin.
Typical Glycan Structures
Glycosylation is an area of post-translational protein modification receiving more attention by researchers as potential cancer biomarkers. Attachment of the sugar chain to the protein determines whether the interaction is deemed N- or O-glycosylation. These interactions of the glycoprotein structure can be represented by symbol nomenclature. Typical glycan structures are shown here.
N-Linked-Glycan

O-Linked-Glycan


NeuAc - N-acetyl-neuraminic acid

Gal - galactose

GlcNAc - N-acetyl-glucosamine

GalNAc - N-acetyl-galactosamine

Fuc - fucose

Man - mannose
Asn - asparagine
Ser - serine
Thr - threonline
Resources
References
- Urlacher, T et al. Glycoprotein Applications Using Near-Infrared Detection Poster presentation, (2012)