Uses of the In-Cell Western™ Assay
In-Cell Western Assays have proven to be a very versatile and efficient tool in studying a variety of areas in life science research.
- Protein Phosphorylation
- Effects of drug compounds on signaling pathways
- Timing/kinetics of signal transduction
- EC50 and IC50 determination
- GPCR activation
- Tau protein accumulation and inhibition
- Gene expression levels
- Virology Assays
- Apoptosis: PARP-1 and Caspase-3 activation
- Cell surface proteins and receptor internalization
- RNAi library screens
- Cell proliferation assays
Protein Phosphorylation
Effects of Drug Compounds on Signaling Pathways
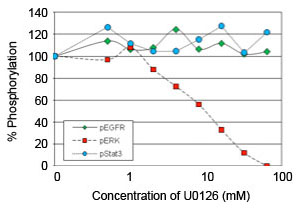
The In-Cell Western assay was used to measure effects of the MEK inhibitor U0126 on EGFR-induced signaling.
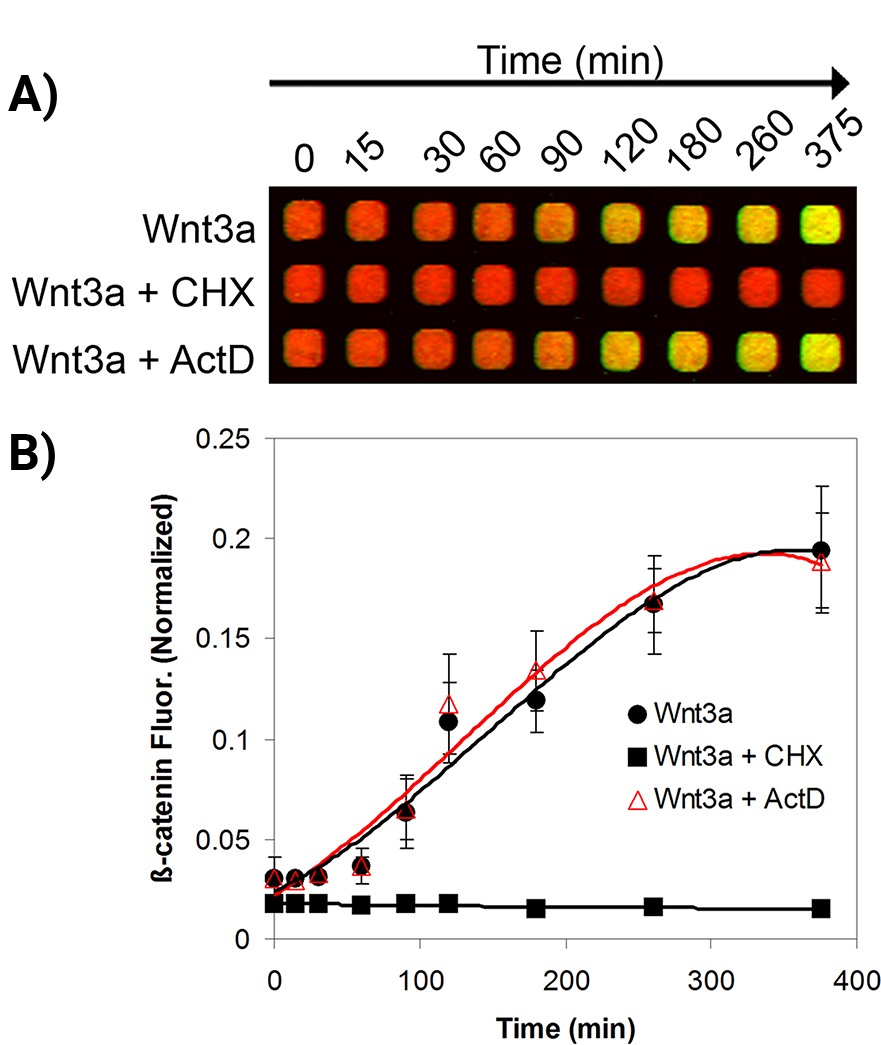
Timing/Kinetics of Signal Transduction
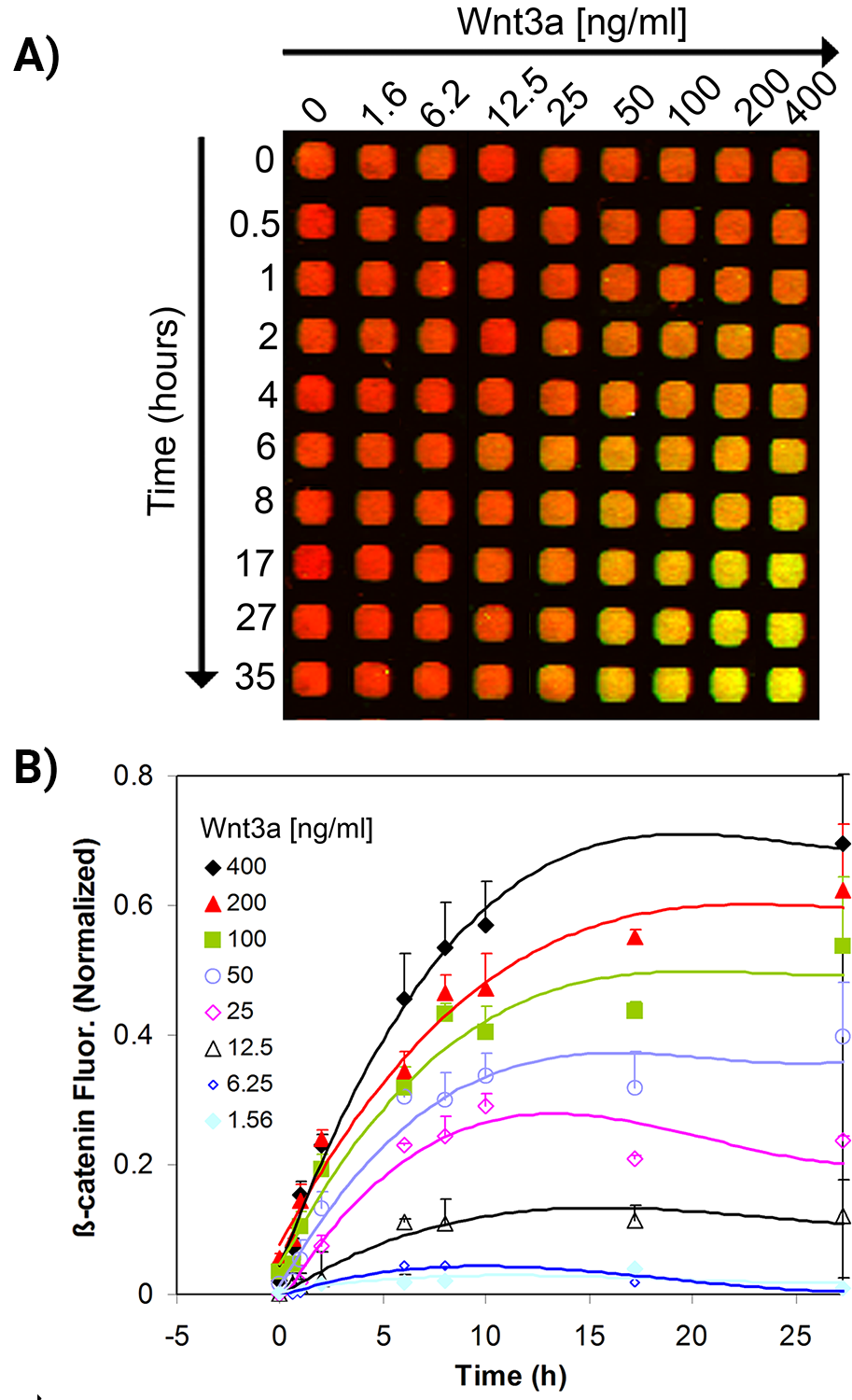
The kinetics of cellular β-catenin accumulation upon stimulation with Wnt3a was studied.
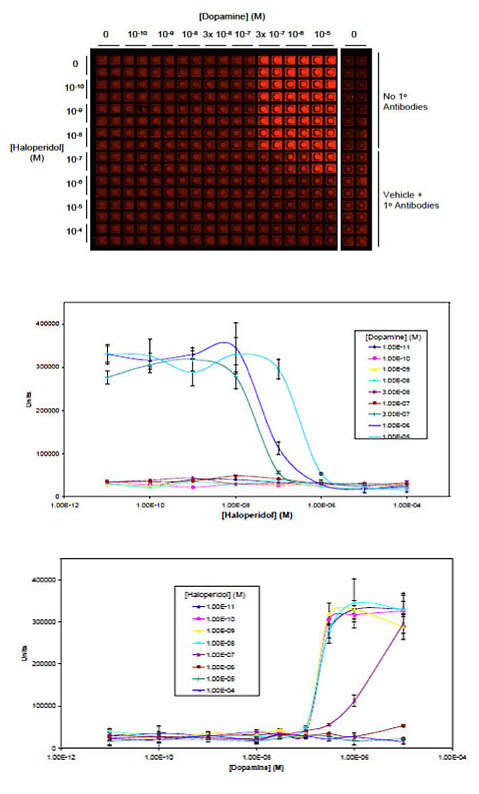
EC50 and IC50 Determinations
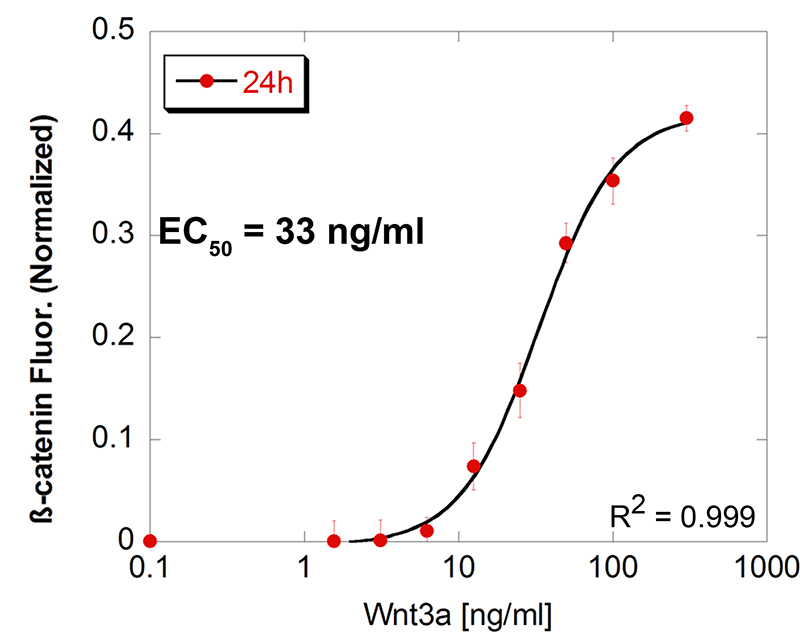
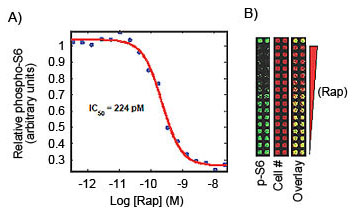
In-Cell Western assays were used to determine EC50 and IC50.
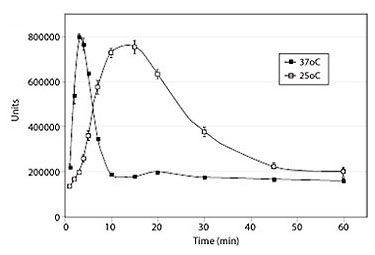
GPCR Activation
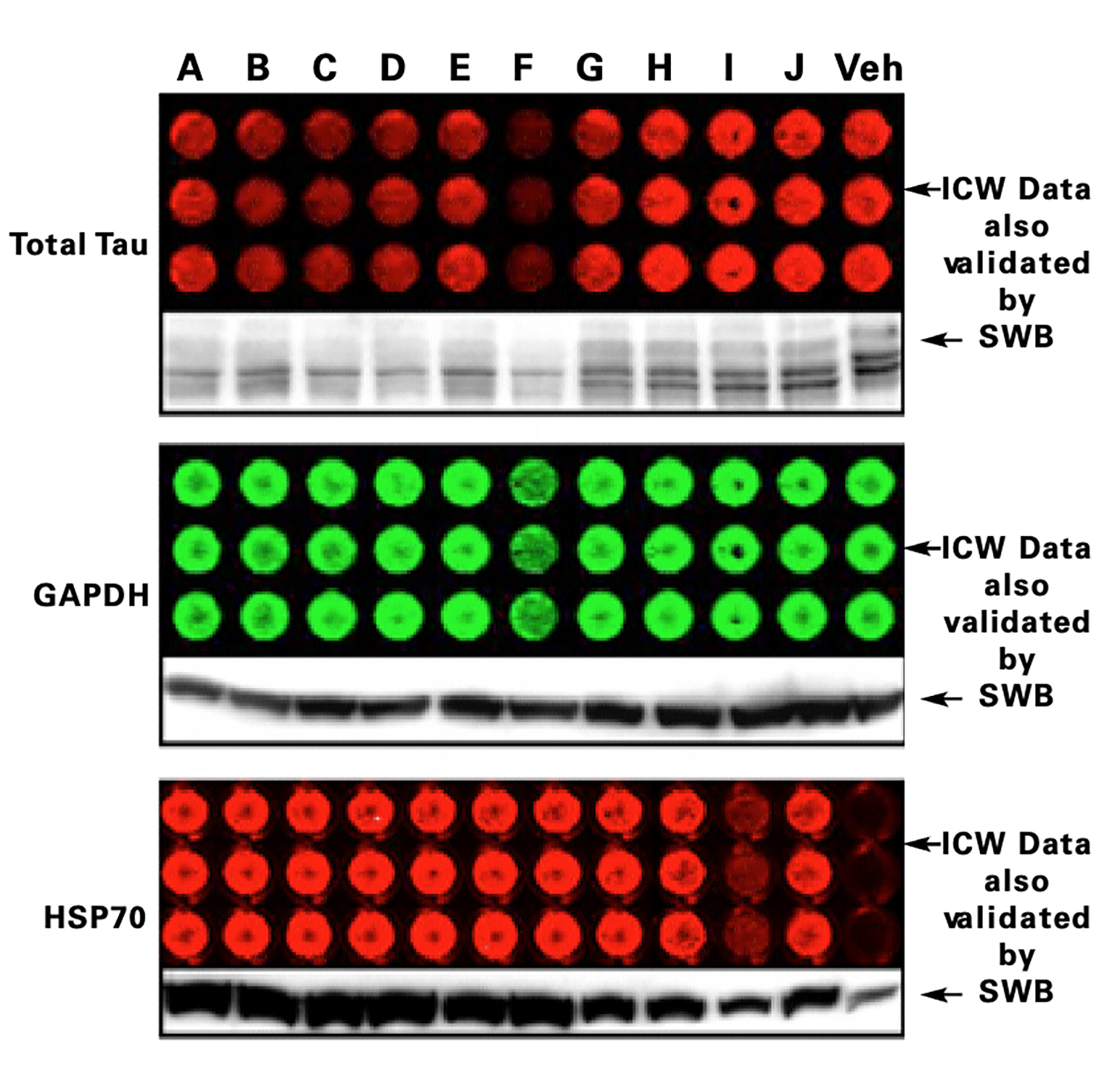
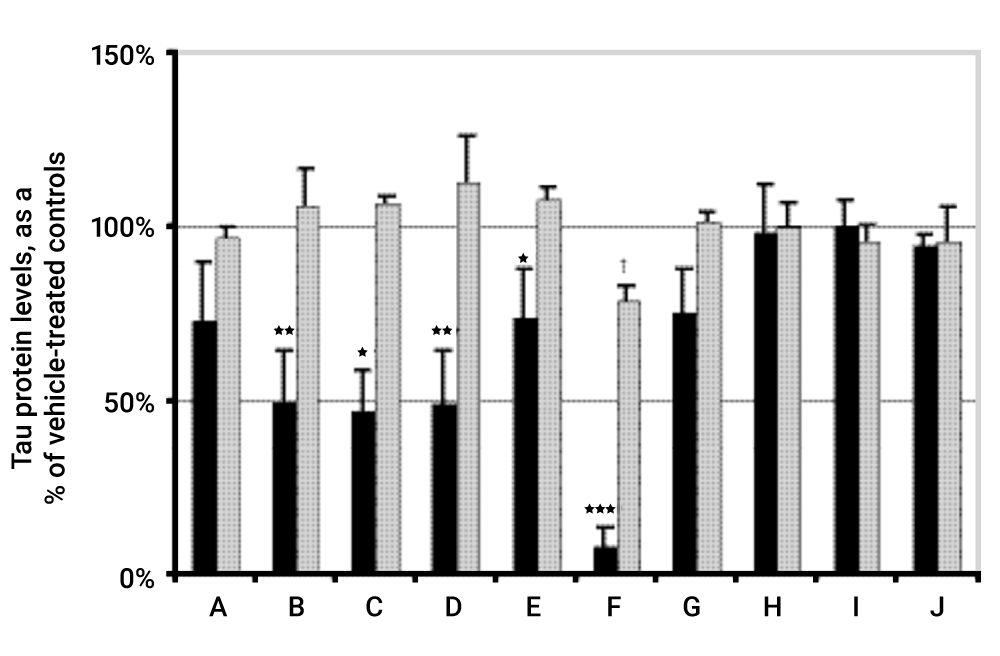
Tau Protein Accumulation and Inhibition
Gene Expression
Virology Assays
The In-Cell Western Assay is a powerful technique for virologists to define specific viral characteristics, such as viral infectivity and mechanism of action, or to screen antiviral therapeutics and test vaccine efficacy. Results from the In-Cell Western have been shown to correlate well with other virology methods and demonstrate that the In-Cell Western is time efficient and well suited for the study of both cytopathic and non-cytopathic viruses.
For instance, when compared with three common methods to quantify Hantaan virus (HTNV) replication: quantitative reverse transcription polymerase chain reaction (qRT-PCR), flow cytometry (FCM), and enzyme-linked immunosorbent assay (ELISA), the In-Cell Western Assay minimized operator subjectivity and was found to have results that were superimposable with results from the other assays.6 This study highlights the capability of the assay for assessing HTNV replication.
For more details about using the In-Cell Western in virology, visit the Virology application page.
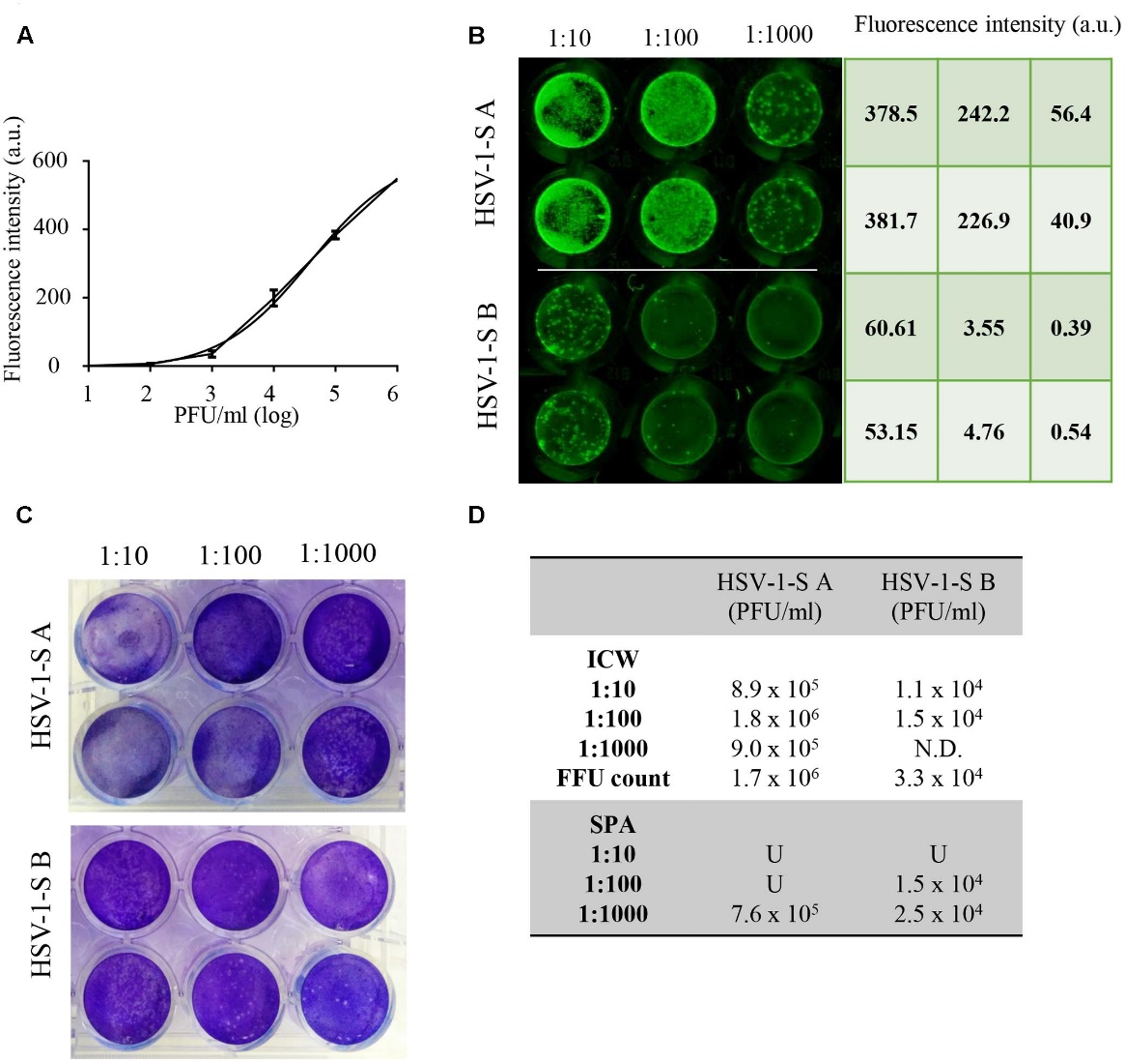
Viral Infectivity
Fabiani, M., et. al. (Fig. 11) found that the In-Cell Western Assay and standard plaque assay (SPA) produced nearly superimposable results. However, the In-Cell Western Assay was faster to detect viral infectivity, enabled many samples to be measured in parallel, and was found to be more suitable for titrations that produced plaques too numerous to count using SPA.7
Cell Surface Proteins
On-Cell Western Assays are a variation of the In-Cell Western Assay in which the cells are not permeabilized. For On-Cell Westerns, the unpermeabilized cells are stained with antibodies against extracellular protein domains so only cell surface antigens are detected. Using On-Cell Western Assays, you can quantitatively monitor cell surface protein expression.
For more information, go to the On-Cell Western Assay application.
RNAi Library Screens
The In-Cell Western Assay can be used in a functional siRNA screen measuring the effects of knockdowns in cultured cells.
For more information and journal references, visit In-Cell Western and RNAi.
Cell Proliferation Assays
Fluorescent cell stains are used for cell proliferation assays for the Odyssey® Imager
SYTO® 60
- Na-Oh Yunn, N-O., et al. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic Acids Res., Sep 2015; 43: 7688–7701.
- Ciaccio, M.F., et al. The DIONESUS algorithm provides scalable and accurate reconstruction of dynamic phosphoproteomic networks to reveal new drug targets. Integr. Biol. (Camb.), Jul 2015; 7: 776–791.
- Yu, N., et al. HSP105 recruits protein phosphatase 2A to dephosphorylate β-catenin. Mol. Cell Biol., Apr 2015; 35: 1390–1400.
- Costa, C., et al. Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell, Jan 2015; 27: 97–108.
- Greenfield, E., Griner, E., and Reproducibility Project: Cancer Biology Registered report: Widespread potential for growth factor-driven resistance to anticancer kinase inhibitors. eLife, Dec 2014; 3: e04037.
- Tunduguru, R., et al. Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells. Biochem. Pharmacol., Nov 2014; 92: 380–388.
- Nolan J. Hoffman, N.J., et al. Chromium Enhances Insulin Responsiveness via AMPK. J. Nutr. Biochem., May 2014; 25: 565–572.
Giemsa Stain
- JP Williams, T Stewart, B Li, R Mulloy, D Dimova, and M Classon. The retinoblastoma protein is required for ras-induced oncogenic transformation. Mol Cell Biol 26(4): 1170-82 (2006)
TO-PRO®-3
- M Quanz, A Herbette, M Sayarath, L de Koning, T Dubois, JS Sun, and M Dutreix. Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci. Mol Cell Biol 26(4): 1170-82 (2006)
See some published examples of In-Cell Western Assays
View publicationsReferences
- Chen, H. et al. (2005). Anal Biochem, 338(1), pp 136-42. DOI: 10.1016/j.ab.2004.11.015.
- Hannoush, R.N. (2008). PLOS ONE, 3(10), e3498. DOI: 10.1371/journal.pone.0003498.
- Hoffman, G.R. et al. (2010). Assay Drug Dev Technol, 8(2), pp 186-99. DOI: 10.1089/adt.2009.0213.
- Wong, S.K. (2004). Anal Biochem, 333(2), pp 265-72. DOI: 10.1016/j.ab.2004.05.011.
- Dickey, C.A. A High Throughput Drug Discovery for Intracellular Neurodegenerative Disease-Associated Targets using the LI-COR Odyssey Near Infrared Imaging System. Research Paper.
- Ma, H.W., et. al. (2017). Frontiers in Cellular and Infection Microbiology, 7(269). DOI: 10.3389/fcimb.2017.00269.
- Fabiani, M., et. al. (2017). Frontiers in Microbiology, 8(1085). DOI: 10.3389/fmicb.2017.01085.
- Weldon, S.K., et. al. (2010). Journal of Virological Methods, 168(1–2), 57–62. DOI: 10.1016/j.jviromet.2010.04.016.
- Sklan, E. H. et. al. (2007). Journal of Virology, 81(20), 11096–11105. DOI: 10.1128/JVI.01249-07.
- Lin, Y.C.J., et. al. (2008). Journal of Virology, 82(12), 5922-5932. DOI: 10.1128/JVI.02723-07.
- Counihan, N.A., et. al. (2006). Journal of Virological Methods, 133(1), 62-69. DOI: 10.1016/j.jviromet.2005.10.023.
