IRDye® 800CW Infrared Dyes
Products
IRDye 800CW near-infrared fluorescent dyes can be used for protein and antibody labeling, or nucleic acid applications with high labeling density.
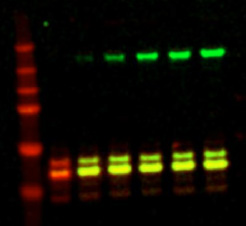
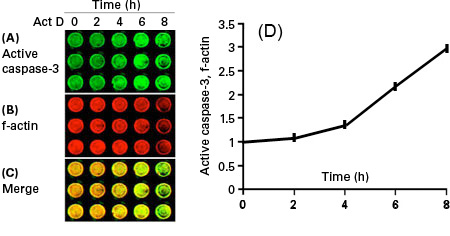
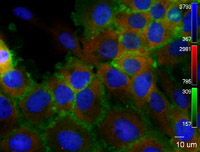
These 800 nm channel near-infrared dyes are superior for Western blotting and immunocytochemical assays, including In-Cell Western™ assays and On-Cell Western cell-based assays, as well as for protein arrays, microscopy, tissue section imaging, and in vivo imaging and optical probe development.
IRDye 800CW dye is characterized by high water solubility and salt tolerance, low non-specific binding to cellular components, and a high signal-to-noise ratio.
IRDye 800CW dye-conjugated agents and probes are currently propelling more than a dozen Phase I or Phase II clinical trials, more than any other near-infrared fluorescent dye.
Other Forms Available
Click Chemistry utilizes pairs of reagents that exclusively react with each other and are effectively inert to naturally occurring functional groups such as amines. LI-COR offers IRDye 800CW DBCO, azide, and alkyne for click chemistry applications.
IRDye 800CW NHS ester is available in labeling kits so that you can label your own compounds for assay or probe development.
IRDye 800CW dye-conjugated forms are available from LI-COR, including:
- Secondary antibody conjugates, including goat and donkey secondary antibodies
- Streptavidin
- Optical imaging agents, including dye-labeled 2-DG, EGF, RGD, BoneTag™, and PEG Fluorescent Contrast Agent
IRDye 800CW Dyes and Conjugates Used in Various Applications